| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Neurokinin-1 receptor
|
|---|---|
| 体外研究 (In Vitro) |
Fosaprepitant (MK-0517, L-758,298) 是阿瑞匹坦的磷酰基前药。皮质类固醇和 5-HT 3 受体拮抗剂被批准与选择性 P 物质(NK-1 受体)拮抗剂阿瑞吡坦联合治疗,以预防化疗引起的急性和迟发性恶心和呕吐。 [1]
|
| 体内研究 (In Vivo) |
福沙匹坦二葡胺(30 mg/kg;腹腔注射;每天;持续7天)在绳中解除对吗啡的耐受性并增加抗损伤作用[1]。 动物模型:Sprague-Dawley大鼠[1] 剂量:30 mg/kg 给药:腹腔注射,每天,持续 7 天 结果:与对照组相比,吗啡的镇痛作用增强。
|
| 动物实验 |
Sprague-Dawley rats were injected with morphine (10 mg/kg twice daily) and/or fosaprepitant (30 mg/kg once daily) for 7 days. Pain threshold was assessed by the hot plate test. Expression of SP and calcitonin gene-related peptide (CGRP) in the spinal cords of these rats was evaluated by immunohistochemistry.[2]
Fosaprepitant (also known as MK-0517 and L-758,298) is a water-soluble phosphoryl prodrug for aprepitant, which, when administered intravenously, is converted to aprepitant within 30 min of intravenous administration via the action of ubiquitous phosphatases. Owing to the rapid conversion of fosaprepitant to the active form (aprepitant), fosaprepitant 115 mg provided the same aprepitant exposure in terms of AUC as aprepitant 12 mg orally, and fosaprepitant is expected to provide a correspondingly similar antiemetic effect as aprepitant. Clinical studies have suggested that fosaprepitant could be appropriate as an intravenous alternative to the aprepitant oral capsule. In a study in healthy subjects, fosaprepitant 115 mg was generally well tolerated at a final drug concentration of 1 mg/ml, and fosaprepitant 115 mg was AUC bioequivalent to aprepitant 125 mg. Fosaprepitant in the dose of 115 mg has been approved by the US FDA, the EU and the Australian authorities on day 1 of a 3-day oral aprepitant regimen, with oral aprepitant administered on days 2 and 3. Fosaprepitant may be a useful parenteral alternative to oral aprepitant. Further study is needed to clarify the utility of fosaprepitant in the prevention of CINV and to clarify optimal dosing regimens that may be appropriate substitutes for oral aprepitant[2]. |
| 参考文献 |
[1]. Expert Opin Investig Drugs. 2007 Dec;16(12):1977-85. [2]. Role of fosaprepitant, a neurokinin Type 1 receptor antagonist, in morphine-induced antinociception in rats. Indian J Pharmacol. 2016 Jul-Aug; 48(4): 394-398.[3]. Fosaprepitant: a neurokinin-1 receptor antagonist for the prevention of chemotherapy-induced nausea and vomiting. Expert Rev Anticancer Ther . 2008 Nov;8(11):1733-42. |
| 其他信息 |
Fosaprepitant Dimeglumine is the dimeglumine salt form of fosaprepitant, the water-soluble, N-phosphorylated prodrug of aprepitant, with antiemetic activity. Upon intravenous administration and rapid conversion to aprepitant, this agent binds selectively to the human substance P/neurokinin 1 (NK1) receptors in the central nervous system (CNS). This inhibits receptor binding of the endogenous substance P and prevents substance P-induced emesis.
|
| 分子式 |
C37H56F7N6O16P
|
|---|---|
| 分子量 |
1004.8337
|
| 精确质量 |
1004.34
|
| 元素分析 |
C, 44.23; H, 5.62; F, 13.23; N, 8.36; O, 25.48; P, 3.08
|
| CAS号 |
265121-04-8
|
| 相关CAS号 |
Fosaprepitant; 172673-20-0; Fosaprepitant-d4 dimeglumine
|
| PubChem CID |
136086851
|
| 外观&性状 |
White solid powder
|
| tPSA |
366.08
|
| 氢键供体(HBD)数目 |
9
|
| 氢键受体(HBA)数目 |
21
|
| 可旋转键数目(RBC) |
13
|
| 重原子数目 |
54
|
| 分子复杂度/Complexity |
1130
|
| 定义原子立体中心数目 |
7
|
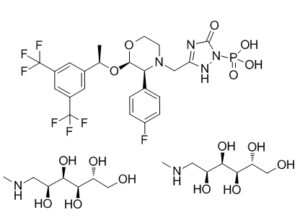
| SMILES |
P(N1C(N([H])C(C([H])([H])N2C([H])([H])C([H])([H])O[C@@]([H])([C@]2([H])C2C([H])=C([H])C(=C([H])C=2[H])F)O[C@]([H])(C([H])([H])[H])C2C([H])=C(C(F)(F)F)C([H])=C(C(F)(F)F)C=2[H])=N1)=O)(=O)(O[H])O[H].O([H])[C@]([H])([C@]([H])(C([H])([H])N([H])C([H])([H])[H])O[H])[C@@]([H])([C@@]([H])(C([H])([H])O[H])O[H])O[H].O([H])[C@]([H])([C@]([H])(C([H])([H])N([H])C([H])([H])[H])O[H])[C@@]([H])([C@@]([H])(C([H])([H])O[H])O[H])O[H]
|
| InChi Key |
VRQHBYGYXDWZDL-OOZCZQCLSA-N
|
| InChi Code |
InChI=1S/C23H22F7N4O6P.2C7H17NO5/c1-12(14-8-15(22(25,26)27)10-16(9-14)23(28,29)30)40-20-19(13-2-4-17(24)5-3-13)33(6-7-39-20)11-18-31-21(35)34(32-18)41(36,37)38;2*1-8-2-4(10)6(12)7(13)5(11)3-9/h2-5,8-10,12,19-20H,6-7,11H2,1H3,(H,31,32,35)(H2,36,37,38);2*4-13H,2-3H2,1H3/t12-,19+,20-;2*4-,5+,6+,7+/m100/s1
|
| 化学名 |
[3-[[(2R,3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)morpholin-4-yl]methyl]-5-oxo-4H-1,2,4-triazol-1-yl]phosphonic acid;(2R,3R,4R,5S)-6-(methylamino)hexane-1,2,3,4,5-pentol
|
| 别名 |
MK0517; MK 0517; MK-0517; Fosaprepitant dimeglumine; Fosaprepitant dimeglumine salt; Ivemend; Fosaprepitant meglumine; MK-0517; Fosaprepitant (dimeglumine); UNII-D35FM8T64X; Emend; Inemend
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: (1). 该产品在溶液状态不稳定,请现配现用。 (2). 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O: ~50 mg/mL (~49.8 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 100 mg/mL (99.52 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.9952 mL | 4.9760 mL | 9.9519 mL | |
| 5 mM | 0.1990 mL | 0.9952 mL | 1.9904 mL | |
| 10 mM | 0.0995 mL | 0.4976 mL | 0.9952 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Efficacy and Safety of Fosaprepitant Dimeglumine in Preventing Chemotherapy-Induced Nausea and Vomiting (MK-0517-031)
CTID: NCT01594749
Phase: Phase 3 Status: Completed
Date: 2018-09-04
|