| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g | |||
| Other Sizes |

| 靶点 |
5-HT3 Receptor ( IC50 = 17 μM )
Granisetron HCl (BRL 43694A) acts as a selective antagonist of 5-hydroxytryptamine 3 (5-HT₃) receptors. In radioligand binding assays using rat brain cortical membranes, it exhibited a high affinity with a Ki value of 0.19 nM for 5-HT₃ receptors, and no significant binding to 5-HT₁, 5-HT₂, dopamine D₂, muscarinic M₁, or opioid μ receptors (Ki > 1000 nM for these receptors) [1] - Granisetron HCl (BRL 43694A) binds to human 5-HT₃A (h5-HT₃A) and human 5-HT₃AB (h5-HT₃AB) receptors expressed in HEK 293 cells, with Ki values of 0.54 nM and 0.67 nM, respectively [5] - Granisetron HCl (BRL 43694A) inhibits 5-HT₃ receptor-mediated responses in NG108-15 cells (a hybrid neuroblastoma-glioma cell line), with an IC₅₀ of 3.2 nM for the suppression of 5-HT-induced calcium ion influx [2] |
|---|---|
| 体外研究 (In Vitro) |
格拉司琼阻断猫离体心室肌细胞的延迟整流电流 (IK),KD 为 4.3 mM。格拉司琼显示出固有的电压依赖性,因为阻滞随着去极化而增加。格拉司琼从细胞内侧的跨膜电场 10% 处的结合位点进行阻断。 Granisetron (3 mM) 可将猫离体心室肌细胞在 0.5 Hz 时的动作电位持续时间 (APD) 延长约 30%。格拉司琼(而非昂丹司琼)可以阻止假定的 5-H 的激活;自身受体,从而导致肠嗜铬细胞释放的血清素减少。细胞测定:在大鼠前胃中,GR 减少 5-HT 诱发的收缩,IC50 为 17 /- 6 uM。在离体兔心脏中,GR 0.003-0.03 nM 剂量依赖性地减少 s-HT 心动过速;高水平的 GR 降低了对 5-HT 的次最大和最大反应。
在大鼠大脑皮层膜制备物中,Granisetron HCl (BRL 43694A) 可浓度依赖性地取代[³H]-昂丹司琼(选择性5-HT₃受体配体),证实其与5-HT₃受体的竞争性结合;在浓度高达10 μM时,其不影响[³H]-5-HT与5-HT₁/₂受体的结合,也不影响[³H]-螺哌隆与多巴胺D₂受体的结合[1] - 在NG108-15细胞中,Granisetron HCl (BRL 43694A) 可浓度依赖性地抑制5-HT诱导的内向电流(通过膜片钳技术记录)和钙离子动员;该抑制作用具有可逆性,且不受5-HT浓度增加的影响(符合功能水平的非竞争性拮抗特征)[2] - 在离体人回肠制备物中,Granisetron HCl (BRL 43694A)(1-100 nM)可抑制5-HT诱导的收缩,在100 nM时抑制率达最大值(92%);在相同浓度下,其对乙酰胆碱或组胺诱导的收缩无影响[3] - 在大鼠原代肝细胞培养中,Granisetron HCl (BRL 43694A) 暴露24 h后,浓度高达10 μM时无显著细胞毒性(通过乳酸脱氢酶释放评估);在50 μM时,细胞毒性仅增加15%[4] - 在表达h5-HT₃A或h5-HT₃AB受体的HEK 293细胞中,Granisetron HCl (BRL 43694A) 可阻断5-HT诱导的全细胞电流(钳位电压-60 mV),对h5-HT₃A和h5-HT₃AB受体的IC₅₀值分别为0.89 nM和1.02 nM,表明其对两种受体亚型的拮抗效力相近[5] |
| 体内研究 (In Vivo) |
格拉司琼对仔猪顺铂引起的呕吐具有显着的益处,一些动物在急性期和延迟期都得到完全保护。每天给予猫 3 次格拉司琼(1 mg/kg,肌肉注射)可显着减少顺铂在第 1 天和第 2 天引起的干呕+呕吐反应,分别达 100.0% 和 75.0%。格拉司琼或地塞米松可显着改善大鼠的宏观和组织学评分,降低髓过氧化物酶活性并降低结肠炎性细胞因子和丙二醛水平。格拉司琼不仅可以防止霍乱毒素诱导的空肠净液分泌,而且还可以按比例抑制 5-HT 释放到小鼠的肠腔中。
在雄性雪貂中,皮下注射(s.c.)Granisetron HCl (BRL 43694A)(0.1-1 mg/kg)可剂量依赖性地抑制顺铂(6 mg/kg,腹腔注射,i.p.)诱导的急性呕吐(顺铂给药后24 h内发生);减少呕吐次数的ED₅₀为0.23 mg/kg,1 mg/kg时可完全抑制呕吐[1] - 在雄性Wistar大鼠中,Granisetron HCl (BRL 43694A)(0.3-3 mg/kg,静脉注射,i.v.)可显著降低吗啡(5 mg/kg,s.c.)诱导的条件性位置偏爱(评估阿片类药物奖赏效应的指标),3 mg/kg时降低幅度达65%;在剂量高达10 mg/kg时,其不影响自发活动[2] - 在雌性Balb/c小鼠中,于环磷酰胺(150 mg/kg,i.p.)给药前30 min口服(p.o.)Granisetron HCl (BRL 43694A)(1 mg/kg),可减轻环磷酰胺诱导的肠黏膜损伤(通过绒毛高度和隐窝深度评估);与仅给予环磷酰胺的组相比,绒毛高度增加28%[3] - 在雄性Sprague-Dawley大鼠中,重复口服Granisetron HCl (BRL 43694A)(10 mg/kg/天,持续28天)对体重增长、食物摄入或器官重量(肝、肾、脾)无显著影响;这些器官未观察到组织病理学改变[4] - 在雄性Sprague-Dawley大鼠中,Granisetron HCl (BRL 43694A)(0.1-1 mg/kg,i.p.)可剂量依赖性地减轻乙酸(0.6%,i.p.)诱导的内脏痛觉过敏(通过腹部收缩次数评估);ED₅₀为0.35 mg/kg,1 mg/kg时收缩次数最大减少72%[5] |
| 酶活实验 |
使用已建立的5-HT3受体活性模型研究了BRL 43694(格拉司琼)的活性。在豚鼠离体回肠中,BRL 43694拮抗了相对高浓度5-羟色胺引起的收缩(pA2=8.1+/-0.2)。然而,除高浓度外,BRL 43694不影响电场刺激(胆碱能介导)、烟碱激动剂二甲基苯基哌嗪(DMPP)或八肽胆囊收缩素诱发的回肠类似制剂的收缩。同样,BRL 43694不影响大鼠或人类离体胃的电诱发、胆碱能介导的收缩。在5-HT3受体活性的其他模型中(兔离体心脏、麻醉大鼠的Bezold-Jarisch反射),BRL 43694显示出强烈的拮抗作用。在大鼠脑膜的放射配体结合研究中,BRL 43694对5-HT1A、5-HT1B、5-HT2或许多其他结合位点几乎没有亲和力。因此,BRL 43694可能是一种强效且选择性的5-HT3受体拮抗剂[Eur J Pharmacol. 1989 Jan 10;159(2):113-24.]。
5-HT₃受体结合实验(大鼠大脑皮层):将新鲜大鼠大脑皮层在冰浴的Tris-HCl缓冲液(50 mM,pH 7.4)中匀浆,48,000 × g离心15 min;重悬沉淀后,与[³H]-昂丹司琼(0.5 nM)及不同浓度的Granisetron HCl (BRL 43694A)(10⁻¹¹-10⁻⁶ M)在25°C孵育60 min。非特异性结合定义为在10 μM昂丹司琼存在下的结合。反应通过玻璃纤维滤膜过滤终止,采用液体闪烁光谱法计数放射性。利用Cheng-Prusoff方程计算Ki值[1] - 5-HT₃受体功能实验(NG108-15细胞):将细胞接种于24孔板,用钙敏感染料Fura-2/AM(5 μM)在37°C负载30 min。洗涤后,细胞与Granisetron HCl (BRL 43694A)(10⁻¹⁰-10⁻⁶ M)孵育10 min,随后加入5-HT(10 μM)。测量荧光强度(激发波长:340/380 nm,发射波长:510 nm)5 min,通过浓度-效应曲线确定IC₅₀值[2] |
| 细胞实验 |
在大鼠前胃中,GR 减少 5-HT 诱发的收缩,IC50 为 17 /- 6 uM。 GR 0.003-0.03 nM 剂量依赖性地降低离体兔心脏中的 s-HT 心动过速;在高浓度下,GR 降低了对 5-HT 的次最大和最大反应。
1.在这项研究中,研究人员研究了两种5-羟色胺3拮抗剂昂丹司琼和格拉司琼对猫离体心室肌细胞动作电位持续时间(APD)和延迟整流电流(IK)的影响。用膜片钳技术在37摄氏度下进行全细胞电流和动作电位记录。2.昂丹司琼和格拉司琼分别以1.7+/-1.0和4.3+/-1.7微M的KD阻断IK。在较高浓度(30微M)下,两种药物都阻断了内向整流器(IKl)。3.IK的阻断依赖于通道激活。这两种药物都减缓了IK尾电流的衰减,并与药物前的电流轨迹产生了交叉。这些结果与通道打开状态下的阻塞和解锁一致。4.格拉司琼显示出内在的电压依赖性,因为随着去极化,阻滞增加。阻断的等效电压依赖性(δ)为0.10+/-0.04,表明格拉司琼在跨膜电场10%的结合位点从细胞内侧阻断。5.昂丹司琼(1微M)和格拉司琼(3微M)在0.5 Hz下将APD延长了约30%。昂丹司琼对APD的延长在更快的频率(3 Hz)下被消除,显示出相反的速率依赖性。6.总之,5-羟色胺3拮抗剂昂丹司琼和格拉司琼是心室延迟整流的开放状态阻断剂,显示出明显的III类作用。 大鼠肝细胞毒性实验:通过胶原酶灌注法分离雄性Wistar大鼠肝细胞,以1×10⁴个细胞/孔接种于96孔板。贴壁24 h后,用Granisetron HCl (BRL 43694A)(0.1-100 μM)处理细胞24 h。收集培养上清液,采用比色法试剂盒测定乳酸脱氢酶(LDH)活性。细胞毒性以LDH释放率表示(相对于用0.1% Triton X-100处理的阳性对照细胞)[4] - h5-HT₃受体电流记录(HEK 293细胞):将转染h5-HT₃A或h5-HT₃AB受体cDNA的HEK 293细胞接种于盖玻片。在室温(22-25°C)下进行全细胞膜片钳记录,细胞外液含140 mM NaCl、5 mM KCl、2 mM CaCl₂、1 mM MgCl₂和10 mM HEPES(pH 7.4)。通过浴灌流方式加入Granisetron HCl (BRL 43694A)(10⁻¹¹-10⁻⁶ M),5 min后加入5-HT(3 μM)。在-60 mV下记录电流幅度,通过非线性回归计算IC₅₀值[5] |
| 动物实验 |
1 mg/kg, i.m. Piglet We analyzed the effects of the 5-HT3 receptor antagonist granisetron on both acute and delayed phases of cisplatin-induced emesis in the conscious piglet. Animals that received a high dose of cisplatin (5.5 mg/kg i.v.) were observed continuously for 60 h. Seventeen piglets were treated with cisplatin only and acted as controls. In experimental animals, granisetron (administered before cisplatin) was administered either as a single initial injection (7 mg/kg), alone or in combination with dexamethasone (40 mg), or as multiple injections (1 mg/kg) given every 5 h during the first 30 h of the experiment (cumulative dose: 7 mg/kg). Two other groups of piglets were treated with dexamethasone (40 mg) alone or with multiple injections of ondansetron (7 injections at 3.5 mg/kg), respectively. The latency to the first emetic episode was significantly increased in all groups that received a 5-HT3 receptor antagonist, whatever the agent and the protocol of administration. Piglets treated solely with dexamethasone exhibited a latency similar to that of controls. The total number of emetic events during the 60 h was significantly reduced only in the group of piglets treated repeatedly with granisetron and in the group that received an initial dose (7 mg/kg) of granisetron in combination with dexamethasone. We observed that 3 out of 8 piglets treated repeatedly with granisetron did not vomit throughout the experiment. These results demonstrate that granisetron, when administered repeatedly, is efficacious against delayed emesis. They also suggest that serotonin may be involved in the production of the delayed phase of cisplatin-induced emesis.[2]
The emetic action of cisplatin was investigated in the cat using a closed circuit video recording system. In initial investigations, cisplatin 3 and 5 mg/kg, i.v. induced emesis over a 2-day period following a latency of 17.6+/-9.6 and 15.6+/-7.8 h, respectively. The anti-emetic efficacy of granisetron and dexamethasone was investigated in the cisplatin 5 mg/kg, i.v.-induced emesis model. In these experiments, cisplatin induced 47.0+/-14.0 and 20.0+/-9.0 retches+vomits on days 1 and 2, respectively, following a latency of 2.4+/-0.4 h. Granisetron (1 mg/kg, i.m.) administered three times per day reduced significantly the retching+vomiting response induced by cisplatin on days 1 and 2 by 100.0% (P<0.05) and 75.0% (P<0.05), respectively; dexamethasone (0.01-1 mg/kg, i.m.) administered three times per day reduced significantly the retching+vomiting response by 68.8-100.0% (P<0.05) and 33.3-100.0% (P<0.05) on days 1 and 2, respectively. The emetic action of cisplatin 7.5 mg/kg, i.v. was also investigated. This dose of cisplatin-induced emesis following a latency of 1.2+/-0.2 h and comprised 119.0+/-20.8 retches+vomits over a 24-h period. Granisetron and dexamethasone antagonized the emesis occurring in the first 3-h period (P<0.05) but were less effective to antagonize the subsequent emetic response (P0.05). The pharmacological sensitivity of low dose cisplatin-induced emesis in the cat is variable but unique and not representative of the clinical situation.[3] Inflammatory bowel disease (IBD) is a chronically relapsing inflammation of the gastrointestinal tract, of which the definite etiology remains ambiguous. Considering the adverse effects and incomplete efficacy of currently administered drugs, it is indispensable to explore new candidates with more desirable therapeutic profiles. 5-HT( 3) receptor antagonists have shown analgesic and anti-inflammatory properties in vitro and in vivo. This study aims to investigate granisetron, a 5-HT( 3) receptor antagonist, in acetic acid-induced rat colitis and probable involvement of 5-HT(3) receptors. Colitis was rendered by instillation of 1 mL of 4% acetic acid (vol/vol) and after 1 hour, granisetron (2 mg/kg), dexamethasone (1 mg/kg), meta-chlorophenylbiguanide (mCPBG, 5 mg/kg), a 5-HT( 3) receptor agonist, or granisetron + mCPBG was given intraperitoneally. Twenty-four hours following colitis induction, animals were sacrificed and distal colons were assessed macroscopically, histologically and biochemically (malondialdehyde, myeloperoxidase, tumor necrosis factor-alpha, interleukin-1 beta and interleukin-6). Granisetron or dexamethasone significantly (p < .05) improved macroscopic and histologic scores, curtailed myeloperoxidase activity and diminished colonic levels of inflammatory cytokines and malondialdehyde. The protective effects of granisetron were reversed by concurrent administration of mCPBG. Our data suggests that the salutary effects of granisetron in acetic acid colitis could be mediated by 5-HT(3) receptors.[4] Ferret anti-emetic assay: Male ferrets (1.0-1.5 kg) were fasted for 18 h before experiments. Granisetron HCl (BRL 43694A) was dissolved in 0.9% saline and administered s.c. at doses of 0.1, 0.3, or 1 mg/kg (volume: 1 mL/kg) 30 min before cisplatin (6 mg/kg, dissolved in 0.9% saline, i.p.). Ferrets were individually housed in observation cages, and the number of vomiting episodes and retches was recorded for 24 h post-cisplatin. A control group received s.c. saline instead of Granisetron HCl (BRL 43694A) [1] - Rat morphine-induced conditioned place preference (CPP) assay: Male Wistar rats (200-250 g) were habituated to a CPP apparatus (two compartments with distinct visual/olfactory cues) for 3 days (15 min/day). During conditioning (days 4-9), rats received Granisetron HCl (BRL 43694A) (0.3, 1, or 3 mg/kg, dissolved in 0.9% saline, i.v.) or saline 10 min before morphine (5 mg/kg, s.c.) and were confined to one compartment for 45 min. On the alternate day, rats received saline (i.v. + s.c.) and were confined to the other compartment. On the test day (day 10), rats were allowed free access to both compartments for 15 min, and time spent in each compartment was recorded. CPP was calculated as the difference in time spent in the morphine-paired compartment between test and habituation days [2] - Rat 28-day repeated toxicity study: Male Sprague-Dawley rats (180-200 g) were randomly divided into 3 groups (n=8/group): control (0.5% methylcellulose, p.o.), low-dose Granisetron HCl (BRL 43694A) (1 mg/kg/day, p.o.), and high-dose Granisetron HCl (BRL 43694A) (10 mg/kg/day, p.o.). Dosing was performed once daily for 28 days. Body weight and food intake were measured weekly. On day 29, rats were euthanized, and blood samples were collected for hematology/biochemistry analysis; liver, kidney, and spleen were excised, weighed, and fixed in 10% formalin for histopathological examination [4] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorption Absorption of is rapid and complete, though oral bioavailability is reduced to about 60% as a result of first pass metabolism. Route of Elimination The remainder of the dose is excreted as metabolites, 48% in the urine and 38% in the feces. Clearance 0.52 L/h/kg [Cancer Patients with 1 mg bid for 7 days] 0.41 L/h/kg [Healthy subject with a single 1 mg dose] Metabolism / Metabolites Primarily hepatic; undergoes N -demethylation and aromatic ring oxidation followed by conjugation. Animal studies suggest that some of the metabolites may have 5-HT 3 receptor antagonist activity. Granisetron has known human metabolites that include 9'-Desmethylgranisetron and 7-Hydroxygranisetron. Biological Half-Life 4-6 hours in healthy patients, 9-12 hours in cancer patients In male Wistar rats, Granisetron HCl (BRL 43694A) (1 mg/kg, i.v.) had a plasma clearance of 12.5 mL/min/kg, a volume of distribution at steady state (Vss) of 1.8 L/kg, and a terminal half-life (t₁/₂) of 2.1 h. After oral administration (5 mg/kg), the absolute bioavailability was 68%, and the peak plasma concentration (Cmax) of 85 ng/mL was reached at 1.2 h (Tmax) [1] - In male beagle dogs, Granisetron HCl (BRL 43694A) (0.5 mg/kg, i.v.) showed a plasma t₁/₂ of 3.5 h, plasma clearance of 9.8 mL/min/kg, and Vss of 2.5 L/kg. Oral administration (2 mg/kg) resulted in a Cmax of 42 ng/mL (Tmax=1.5 h) and absolute bioavailability of 52% [4] - In human volunteers (n=6), Granisetron HCl (BRL 43694A) (1 mg, i.v.) had a plasma t₁/₂ of 6.2 h, plasma clearance of 8.3 mL/min/kg, and Vss of 3.6 L/kg. Oral administration (2 mg) yielded a Cmax of 3.8 ng/mL (Tmax=2.0 h) and absolute bioavailability of 60% [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the use of granisetron during breastfeeding. Until more data become available, granisetron should be used with caution during breastfeeding. An alternate drug may be preferred. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk A woman nursing an 8-month-old infant 6 to 8 times daily was admitted to the hospital for an appendectomy. During the procedure she received granisetron, cefazolin, ketorolac, rocuronium, succinylcholine, and sufentanil. The patient also received 2 boluses of intravenous propofol of 150 mg followed shortly thereafter by a 50 mg dose. Postoperatively, she was receiving acetaminophen, cefazolin, ibuprofen, and pantoprazole, as well as oxycodone and dimenhydrinate as needed. Twenty-two hours after the procedure, the mother extracted milk for the first time and noted it to be light green in color. Analysis of the green milk using a nonvalidated assay detected no propofol. The green color faded and was absent by postoperative day 4 when she resumed breastfeeding. The authors judged that the green color was possibly caused by propofol or one of its metabolites. In male Sprague-Dawley rats, the no-observed-adverse-effect level (NOAEL) of Granisetron HCl (BRL 43694A) was 10 mg/kg/day (p.o.) for 28 days, as no significant changes in hematology (red blood cell count, white blood cell count, hemoglobin), serum biochemistry (alanine transaminase, aspartate transaminase, creatinine), or organ histopathology were observed at this dose [4] - In male beagle dogs, repeated oral administration of Granisetron HCl (BRL 43694A) (5 mg/kg/day for 28 days) caused a slight increase (12%) in serum creatinine, but no histopathological changes in the kidney; the NOAEL was 2 mg/kg/day [4] - The plasma protein binding rate of Granisetron HCl (BRL 43694A) in human plasma (measured via ultrafiltration) was 65-70% at concentrations of 10-1000 ng/mL, with no concentration-dependent changes in binding [4] - In female Balb/c mice, Granisetron HCl (BRL 43694A) (up to 10 mg/kg, p.o.) did not induce acute toxicity (mortality, convulsions, or significant behavioral changes) within 72 h of administration [3] |
| 参考文献 | |
| 其他信息 |
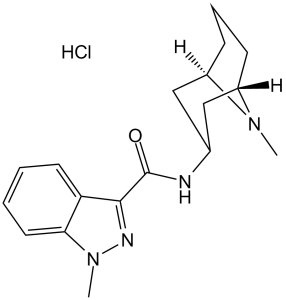
Granisetron hydrochloride is an aromatic amide and a member of indazoles.
A serotonin receptor (5HT-3 selective) antagonist that has been used as an antiemetic for cancer chemotherapy patients. See also: Granisetron Hydrochloride (annotation moved to). Drug Indication Prevention of nausea and vomiting in patients receiving moderately or highly emetogenic chemotherapy, with or without cisplatin, for up to five consecutive days. Sancuso may be used in patients receiving their first chemotherapy regimen or in patients who have previously received chemotherapy. Granisetron HCl (BRL 43694A) is a second-generation 5-HT₃ receptor antagonist developed for the prevention of chemotherapy-induced nausea and vomiting (CINV), with higher selectivity and longer duration of action compared to first-generation antagonists (e.g., ondansetron) [1] - The antagonistic effect of Granisetron HCl (BRL 43694A) on 5-HT₃ receptors is thought to involve blocking 5-HT release from enterochromaffin cells in the gastrointestinal tract (triggered by chemotherapy), thereby preventing activation of vagal afferents and subsequent emetic signals to the brainstem [3] - Granisetron HCl (BRL 43694A) shows no significant interaction with cytochrome P450 enzymes (CYP1A2, CYP2C9, CYP2D6, CYP3A4) in human liver microsomes, indicating a low risk of drug-drug interactions with medications metabolized by these enzymes [4] |
| 分子式 |
C18H25CLN4O
|
|---|---|
| 分子量 |
348.87
|
| 精确质量 |
348.171
|
| 元素分析 |
C, 61.97; H, 7.22; Cl, 10.16; N, 16.06; O, 4.59
|
| CAS号 |
107007-99-8
|
| 相关CAS号 |
Granisetron; 109889-09-0; 107007-99-8 (HCl)
|
| PubChem CID |
6918003
|
| 外观&性状 |
White solid powder
|
| 密度 |
1.33g/cm3
|
| 沸点 |
532ºC at 760mmHg
|
| 熔点 |
290-292°C
|
| 闪点 |
275.6ºC
|
| 蒸汽压 |
0mmHg at 25°C
|
| 折射率 |
1.69
|
| LogP |
3.449
|
| tPSA |
50.16
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
442
|
| 定义原子立体中心数目 |
2
|
| SMILES |
Cl[H].O=C(C1C2=C([H])C([H])=C([H])C([H])=C2N(C([H])([H])[H])N=1)N([H])C1([H])C([H])([H])[C@@]2([H])C([H])([H])C([H])([H])C([H])([H])[C@@]([H])(C1([H])[H])N2C([H])([H])[H]
|
| InChi Key |
QYZRTBKYBJRGJB-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C18H24N4O.ClH/c1-21-13-6-5-7-14(21)11-12(10-13)19-18(23)17-15-8-3-4-9-16(15)22(2)20-17;/h3-4,8-9,12-14H,5-7,10-11H2,1-2H3,(H,19,23);1H
|
| 化学名 |
1-methyl-N-(9-methyl-9-azabicyclo[3.3.1]nonan-3-yl)indazole-3-carboxamide;hydrochloride
|
| 别名 |
BRL43694; BRL 43694; BRL43694A; BRL 43694A; BRL-43694; BRL-43694A; Granisetron Hydrochloride; Granisetron hydrocholride,(S); 1-methyl-N-((1R,3r,5S)-9-methyl-9-azabicyclo[3.3.1]nonan-3-yl)-1H-indazole-3-carboxamide hydrochloride; Granisetron HCl; GRAN; US trade name: Kytril
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 0.77 mg/mL (2.21 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 7.7 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 0.77 mg/mL (2.21 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 7.7 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 View More
配方 3 中的溶解度: 100 mg/mL (286.64 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8664 mL | 14.3320 mL | 28.6640 mL | |
| 5 mM | 0.5733 mL | 2.8664 mL | 5.7328 mL | |
| 10 mM | 0.2866 mL | 1.4332 mL | 2.8664 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05325190 | Recruiting | Drug: Granisetron Transdermal Patch System |
Chemotherapy-induced Nausea and Vomiting |
Tianjin Medical University Cancer Institute and Hospital |
October 10, 2021 | Phase 2 |
| NCT04472143 | Recruiting | Drug: Granisetron Transdermal Other: 0.9% normal saline |
Granisetron | Assiut University | April 2022 | Phase 2 Phase 3 |
| NCT05314257 | Recruiting | Drug: Granisetron Hydrochloride Behavioral: Resistance training |
Histamine | University Ghent | September 1, 2023 | Not Applicable |
| NCT03817970 | Recruiting | Drug: Granisetron Drug: Ondansetron Drug: Palonosetron |
Nephrotoxicity | University of Colorado, Denver | November 15, 2019 | Phase 3 |
| NCT00873197 | Completed | Drug: granisetron | Healthy | Prostrakan Pharmaceuticals | April 2009 | Phase 1 |
 |
|---|
 |
 |