| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |

| 靶点 |
5-HT3 Receptor ( IC50 = 17 μM )
|
|---|---|
| 体外研究 (In Vitro) |
GR 在大鼠前胃中的 IC50 为 17/± 6 uM,可减少 5-HT 诱导的收缩。 GR 以剂量依赖性方式降低离体兔心脏的 s-HT 心动过速,范围为 0.003-0.03 nM;高 GR 水平还会降低对 5-HT 的次最大和最大反应 [1]。
|
| 体内研究 (In Vivo) |
炎症发生后 6 小时和 72 小时,格拉司琼剂量依赖性地减少白细胞积累。格拉司琼在较低剂量(50μg/袋)时升高PGE(2)水平,但在较高剂量(100和200μg/袋)时释放受到抑制。同时,较低剂量的格拉司琼会减少 TNFα 的产生,但较高剂量的格拉司琼会增加 TNFα 的产生;这两种效应是相互影响的[2]。事实证明,GTDS 并不逊色于口服格拉司琼:65% 接受口服格拉司琼的患者和 60% 接受 GTDS 的患者获得完全控制(治疗差异,-5%;95% 置信范围,-13-3)。便秘是两种耐受性良好的疗法最常见的副作用[3]。
|
| 酶活实验 |
1044/10000
实时翻译
划译
使用已建立的5-HT3受体活性模型研究了BRL 43694(格拉司琼)的活性。在豚鼠离体回肠中,BRL 43694拮抗了相对高浓度5-羟色胺引起的收缩(pA2=8.1+/-0.2)。然而,除高浓度外,BRL 43694不影响电场刺激(胆碱能介导)、烟碱激动剂二甲基苯基哌嗪(DMPP)或八肽胆囊收缩素诱发的回肠类似制剂的收缩。同样,BRL 43694不影响大鼠或人类离体胃的电诱发、胆碱能介导的收缩。在5-HT3受体活性的其他模型中(兔离体心脏、麻醉大鼠的Bezold-Jarisch反射),BRL 43694显示出强烈的拮抗作用。在大鼠脑膜的放射配体结合研究中,BRL 43694对5-HT1A、5-HT1B、5-HT2或许多其他结合位点几乎没有亲和力。因此,BRL 43694可能是一种强效且选择性的5-HT3受体拮抗剂[1]。
|
| 动物实验 |
The antagonists of 5HT(3) receptors have shown impressive efficacy in rheumatoid arthritis, osteoarthritis or fibromyalgia. The mechanistic relationships between 5HT(3) receptors, angiogenesis and sequence of cytokine expression, and leukocyte recruitment during inflammation are not clear. We evaluate the effects of granisetron on inflammatory parameters and angiogenesis in rat air-pouch model.
Methods: Male Wistar rats were anesthetized, and then 20 ml and 10 ml of sterile air were injected subcutaneously in the back on day 0 and day 3, respectively. On day 6, inflammation was induced by injection of 1 ml of carrageenan 1% into pouches. After 6 and 72 h, the rats were sacrificed; pouch fluid was collected in order to determine exudate volume, the number of accumulated cells and TNFalpha/PGE(2) concentration. Pouches were dissected out and weighed. Angiogenesis of granulomatous tissue was assayed using a hemoglobin kit.
Results: Leukocyte accumulation was dose-dependently inhibited by granisetron both at 6 and 72 h after induction of inflammation. All doses of granisetron decreased hemoglobin level in the whole granulation tissue in a bell-shaped manner. Vascular network formation was also inhibited by granisetron. Granisetron increased PGE(2) level at a lower dose (50 microg/pouch) but higher doses (100 and 200 microg/pouch) inhibited the release. At the same time, TNFalpha production was decreased by the lower dose and increased by higher doses of granisetron in a reciprocal fashion.
Conclusions: Anti-inflammatory activities of 5HT(3) receptor antagonist, granisetron probably are mediated through modulation of TNFalpha/PGE(2) production and leukocyte infiltration[2].
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorption of is rapid and complete, though oral bioavailability is reduced to about 60% as a result of first pass metabolism. The remainder of the dose is excreted as metabolites, 48% in the urine and 38% in the feces. 0.52 L/h/kg [Cancer Patients with 1 mg bid for 7 days] 0.41 L/h/kg [Healthy subject with a single 1 mg dose] Metabolism / Metabolites Primarily hepatic; undergoes N -demethylation and aromatic ring oxidation followed by conjugation. Animal studies suggest that some of the metabolites may have 5-HT 3 receptor antagonist activity. Granisetron has known human metabolites that include 7-Hydroxygranisetron and 9'-Desmethylgranisetron. Biological Half-Life 4-6 hours in healthy patients, 9-12 hours in cancer patients |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the use of granisetron during breastfeeding. Until more data become available, granisetron should be used with caution during breastfeeding. An alternate drug may be preferred. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk A woman nursing an 8-month-old infant 6 to 8 times daily was admitted to the hospital for an appendectomy. During the procedure she received granisetron, cefazolin, ketorolac, rocuronium, succinylcholine, and sufentanil. The patient also received 2 boluses of intravenous propofol of 150 mg followed shortly thereafter by a 50 mg dose. Postoperatively, she was receiving acetaminophen, cefazolin, ibuprofen, and pantoprazole, as well as oxycodone and dimenhydrinate as needed. Twenty-two hours after the procedure, the mother extracted milk for the first time and noted it to be light green in color. Analysis of the green milk using a nonvalidated assay detected no propofol. The green color faded and was absent by postoperative day 4 when she resumed breastfeeding. The authors judged that the green color was possibly caused by propofol or one of its metabolites. Protein Binding 65% |
| 参考文献 |
|
| 其他信息 |
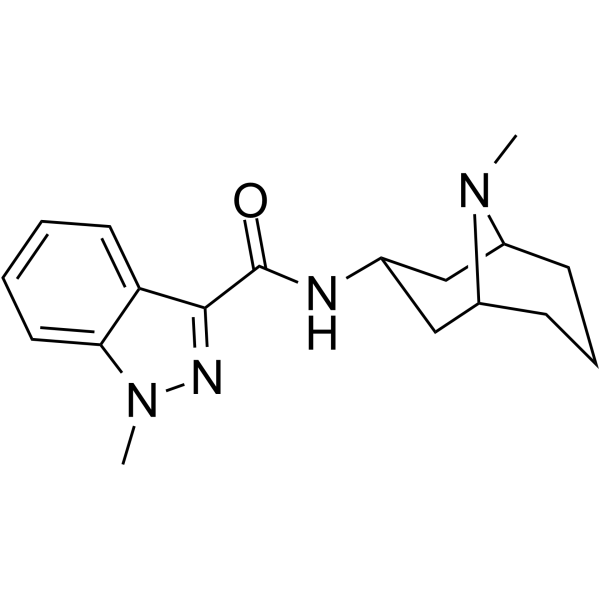
Granisetron is a monocarboxylic acid amide resulting from the formal condensation of the carboxy group of 1-methyl-1H-indazole-3-carboxylic acid with the primary amino group of (3-endo)-9-methyl-9-azabicyclo[3.3.1]nonan-3-amine. A selective 5-HT3 receptor antagonist, it is used (generally as the monohydrochloride salt) to manage nausea and vomiting caused by cancer chemotherapy and radiotherapy, and to prevent and treat postoperative nausea and vomiting. It has a role as a serotonergic antagonist and an antiemetic. It is a member of indazoles, a monocarboxylic acid amide and a tertiary amino compound.
A serotonin receptor (5HT-3 selective) antagonist that has been used as an antiemetic and antinauseant for cancer chemotherapy patients. Granisetron is a Serotonin-3 Receptor Antagonist. The mechanism of action of granisetron is as a Serotonin 3 Receptor Antagonist. Granisetron is an indazole derivative with antiemetic properties. As a selective serotonin receptor antagonist, granisetron competitively blocks the action of serotonin at 5-hydroxytryptamine3 (5-HT3) receptors, resulting in the suppression of chemotherapy- and radiotherapy-induced nausea and vomiting. APF530 is a controlled-release formulation of a biodegradable poly(ortho ester) polymer, encapsulating the indazole derivative granisetron, with antiemetic activity. Upon administration of APF530, the polymer slowly erodes and releases the active ingredient granisetron. As a selective serotonin receptor antagonist, granisetron competitively blocks the action of serotonin at 5-hydroxytryptamine3 (5-HT3) receptors, resulting in the suppression of nausea and vomiting over a sustained period of time. A serotonin receptor (5HT-3 selective) antagonist that has been used as an antiemetic for cancer chemotherapy patients. See also: Granisetron (annotation moved to). Drug Indication For the prevention of nausea and vomiting associated with initial and repeat courses of emetogenic cancer therapy (including high dose cisplatin), postoperation, and radiation (including total body irradiation and daily fractionated abdominal radiation). FDA Label Prevention of nausea and vomiting in patients receiving moderately or highly emetogenic chemotherapy, with or without cisplatin, for up to five consecutive days. Sancuso may be used in patients receiving their first chemotherapy regimen or in patients who have previously received chemotherapy. Mechanism of Action Granisetron is a potent, selective antagonist of 5-HT3 receptors. The antiemetic activity of the drug is brought about through the inhibition of 5-HT3 receptors present both centrally (medullary chemoreceptor zone) and peripherally (GI tract). This inhibition of 5-HT3 receptors in turn inhibits the visceral afferent stimulation of the vomiting center, likely indirectly at the level of the area postrema, as well as through direct inhibition of serotonin activity within the area postrema and the chemoreceptor trigger zone. Pharmacodynamics Granisetron is a selective inhibitor of type 3 serotonergic (5-HT3) receptors. Granisetron has little or no affinity for other serotonin receptors, including 5-HT 1 , 5-HT 1A , 5-HT 1B/C , or 5-HT 2 ; for alpha 1 -, alpha 2 -, or beta-adrenoreceptors; for dopamine D 2 receptors; for histamine H 1 receptors; for benzodiazepine receptors; for picrotoxin receptors; or for opioid receptors. In most human studies, granisetron has had little effect on blood pressure, heart rate, or electrocardiogram (ECG). The drug is structurally and pharmacologically related to ondansetron, another selective inhibitor of 5-HT3 receptors. The serontonin 5-HT3 receptors are located on the nerve terminals of the vagus in the periphery, and centrally in the chemoreceptor trigger zone of the area postrema. The temporal relationship between the emetogenic action of emetogenic drugs and the release of serotonin, as well as the efficacy of antiemetic agents suggest that chemotherapeutic agents release serotonin from the enterochromaffin cells of the small intestine by causing degenerative changes in the GI tract. The serotonin then stimulates the vagal and splanchnic nerve receptors that project to the medullary vomiting center, as well as the 5-HT3 receptors in the area postrema, thus initiating the vomiting reflex, causing nausea and vomiting. |
| 分子式 |
C18H24N4O
|
|---|---|
| 分子量 |
312.40936
|
| 精确质量 |
312.195
|
| 元素分析 |
C, 69.20; H, 7.74; N, 17.93; O, 5.12
|
| CAS号 |
109889-09-0
|
| 相关CAS号 |
Granisetron Hydrochloride;107007-99-8;Granisetron-d3;1224925-64-7
|
| PubChem CID |
5284566
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
532.0±40.0 °C at 760 mmHg
|
| 闪点 |
275.6±27.3 °C
|
| 蒸汽压 |
0.0±1.4 mmHg at 25°C
|
| 折射率 |
1.690
|
| LogP |
1.47
|
| tPSA |
50.16
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
23
|
| 分子复杂度/Complexity |
442
|
| 定义原子立体中心数目 |
2
|
| SMILES |
CN1[C@@H]2CCC[C@H]1CC(C2)NC(=O)C3=NN(C4=CC=CC=C43)C
|
| InChi Key |
MFWNKCLOYSRHCJ-AGUYFDCRSA-N
|
| InChi Code |
InChI=1S/C18H24N4O/c1-21-13-6-5-7-14(21)11-12(10-13)19-18(23)17-15-8-3-4-9-16(15)22(2)20-17/h3-4,8-9,12-14H,5-7,10-11H2,1-2H3,(H,19,23)/t12?,13-,14+
|
| 化学名 |
1-methyl-N-[(1R,5S)-9-methyl-9-azabicyclo[3.3.1]nonan-3-yl]indazole-3-carboxamide
|
| 别名 |
granisetron; 109889-09-0; Sancuso; Sustol; Kevatril; BRL-43694; Granisetronum; APF530;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~25 mg/mL (~80.02 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.00 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.00 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (8.00 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.2009 mL | 16.0046 mL | 32.0092 mL | |
| 5 mM | 0.6402 mL | 3.2009 mL | 6.4018 mL | |
| 10 mM | 0.3201 mL | 1.6005 mL | 3.2009 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。