| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
hLXRα (EC50 = 190 nM); hLXRβ(EC50 = 30 nM)[4]
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
在体外,GW3965 盐酸盐可诱导 GBM 细胞死亡,并且对表达 EGFRvIII 的肿瘤细胞具有更高的效力。 GW3965 盐酸盐可降低 LDLR 水平,同时上调 E3 泛素连接酶 IDOL 和胆固醇转运蛋白基因 ABCA1 的表达[2]。 LXR 配体抑制胶原蛋白或 CRP 诱导的血小板聚集和钙动员。当用 1 μg/mL CRP 激活血小板时,GW3965 盐酸盐(1 或 5 μM)对纤维蛋白原结合和 P-选择素暴露表现出轻微的抑制作用。然而,较高浓度的 GW3965 盐酸盐 (10 μM) 和 T0901317 (40 μM) 会减少血小板表面纤维蛋白原和 P-选择素的量[3]。
LXR激动剂GW3965在体外促进GBM细胞死亡,增强表达EGFRvIII的肿瘤细胞的疗效[2] 细胞内胆固醇水平可以通过以下方式调节:(1)通过LDLR摄取LDL,(2)通过ABCA1或ABCG1转运蛋白排出胆固醇,以及(3)羟甲基戊二酰辅酶A还原酶(HMG-CoA还原酶)依赖性合成。鉴于药物LXR激活限制细胞内胆固醇可用性的能力,我们假设合成LXR激动剂可能会抑制GBM细胞的生长和存活。 事实上,用LXR激动剂GW3965治疗U87和U87 EGFRvIII GBM细胞4天,导致剂量依赖性生长抑制和促进肿瘤细胞死亡。此外,与携带EGFRvIII的肿瘤细胞对外源性胆固醇的依赖性增强一致,这些细胞的细胞死亡明显大于亲本U87细胞系(图4A-D)。值得注意的是,添加LDL可以剂量依赖性地挽救肿瘤细胞死亡(图4E-G),这强烈表明GW3965的杀瘤作用是通过改变细胞胆固醇的可用性来介导的。[2] 为了揭示GW3965诱导肿瘤细胞死亡的机制,对LXR靶基因ABCA1和IDOL进行了实时PCR和免疫印迹分析GW3965治疗促进ABCA1和IDOL的剂量依赖性增加,同时LDLR蛋白水平降低(图5A-D;补充图S5)。不幸的是,目前还没有能够检测内源性IDOL表达的抗体。通过ABCA1调节胆固醇流出是一个1步过程;ABCA1是LXR的直接转录靶点。相比之下,LXR对LDLR的调控需要IDOL的转录和翻译,然后是泛素介导的LDLR降解。GW3965介导的LDLR在GBM细胞中的降解需要更长的时间,并且需要比ABCA1诱导更高的药物剂量(图5C和D)。GW3965对ABCA1和LDLR表达的影响在一组GBM和其他癌症细胞系中得到证实,这些细胞系的LDLR水平与高水平的EGFR磷酸化有关(图5E)。值得注意的是,促进细胞死亡所需的GW3965剂量(图4B-D)与完成LDLR降解所需的剂量(图5C和D)密切相关。总之,这些结果表明,GW3965的杀瘤活性需要降低LDLR水平。[2] 为了直接测试GBM细胞死亡是否需要LDLR降解来响应GW3965,我们测量了慢病毒LDLR shRNA敲除或打乱对照对药物敏感性的影响。低剂量GW3965(1或2μM)诱导ABCA1,但不会降低LDLR表达或导致GBM细胞死亡(图6A-C)。慢病毒递送LDLR shRNA导致LDLR敲除,在低剂量GW3965治疗后,有力地促进了肿瘤细胞死亡(图6A-C)。为了研究IDOL介导的LDLR降解在促进这种凋亡反应中的作用(图6D),我们测量了腺病毒递送IDOL对低剂量GW3965致敏U87 EGFRvIII GBM细胞的影响。酚复制LDLR敲除的效果,IDOL过表达能有效地使GBM细胞对低剂量GW3965敏感(图6E和F)。单独的LDLR敲除和单独的IDOL过表达都不足以促进GBM细胞死亡(图6)。这些发现表明,IDOL介导的LDLR降解是GW3965诱导GBM细胞死亡机制的重要组成部分。然而,仅靶向LDLR不足以引发GBM细胞死亡的观察表明,其他机制,如促进ABCA1依赖性胆固醇外流,也有助于[2]。 GW3965导致LXR与胶原受体GPVI近端的信号成分相关,这表明LXR在血小板中的作用可能导致血小板反应减弱。动脉粥样硬化病变部位血小板的激活会导致心肌梗死和中风前的血栓形成。使用小鼠体内血栓形成模型,我们发现GW3965具有抗血栓形成作用,可以减小血栓的大小和稳定性。GW3965的动脉粥样硬化保护作用及其新的抗血小板/血栓形成作用表明,LXR是预防动脉粥样硬化血栓形成疾病的潜在靶点。[3] 为了进一步提高叔胺的LXRα活性,使用Rink酰胺接头合成了1280种羧酰胺,并在1μM下筛选了LXRα/SRC1-LiSA的活性。从阵列中鉴定出六种羧酰胺(6−11),其在LXRα/SRC1-LiSA中的活性小于1μM(表1)。其中一些类似物在苄胺取代基(9−11)中含有间三氟甲基官能团,其中2-氯-3-三氟甲基苄胺11被确定为最有效的类似物,在LXRα/SRC1-LiSA中的EC50为45 nM。如早期系列羧酰胺所示,11在基于细胞的LXRα-GAL4报告基因检测中显示效力降低。由于通过酰胺4转化为羧酸5提高了细胞效力,因此使用Sasrin接头合成了相应的羧酸12。羧酸12/GW3965在LXRα/SRC1-LiSA中的EC50为125 nM,在SRC1肽的募集方面与EPC(1)具有相当的疗效。令我们高兴的是,GW3965/12在基于LXRα细胞的报告基因测定中保持了其效力,EC50为190 nM(表1,图1B)。当针对一组核受体进行筛选时,12个仅与LXRβ(图1A)和孕烷X受体(PXR)表现出交叉反应性(数据未显示)。LXRβ-和PXR-GAL4嵌合体的全剂量反应分析表明,与PXR相比,12对LXR的激活选择性是10倍以上(图1B)。因此,羧酸12是一种强效的LXR激动剂,具有良好的细胞活性和对其他核受体的优异选择性[4]。 |
||
| 体内研究 (In Vivo) |
非病理动物的 CNS 不会经历 GW3965 盐酸盐引起 STZ 大鼠脊髓、小脑和大脑皮层神经活性类固醇的升高。当糖尿病动物接受GW3965盐酸盐治疗时,它们的脊髓表达更多的髓磷脂碱性蛋白,并具有更高水平的二氢孕酮[1]。在体内,GW3965 盐酸盐(40 mg/kg,口服)可显着使 GBM 细胞死亡增加 25 倍,并强力促进 ABCA1 并降低 LDLR 表达[2]。此外,它还能抑制 59% 的肿瘤生长。 GW3965盐酸盐(2mg/kg,IV)可延长体内出血持续时间并调节血小板血栓形成[3]。
观察到Ro5-4864(即TSPO的配体)或GW3965(即LXRs的配体)治疗诱导STZ大鼠脊髓、小脑和大脑皮层中神经活性类固醇的增加,但在非病理动物的CNS中没有。有趣的是,所分析的三个中枢神经系统区域和两种药理学工具之间的诱导模式不同。特别是,LXRs的激活可能代表了一种有前景的神经保护策略,因为与Ro5-4864治疗不同,GW3965治疗没有引起神经活性类固醇血浆水平的显著变化。这表明,激活LXRs可能会选择性地提高中枢神经系统中神经活性类固醇的水平,从而避免这些分子全身治疗可能产生的内分泌副作用。有趣的是,GW3965治疗诱导糖尿病动物脊髓中二氢孕酮的增加,这与髓鞘碱性蛋白表达的增加有关。因此,我们证明LXR激活能够缓解糖尿病的中枢神经系统症状。[1] LXR激动剂在体内抑制GBM肿瘤生长[2] 为了测试LXR激动剂在治疗GBM方面的治疗潜力,我们确定了GW3965在体内阻断生长和促进肿瘤细胞死亡的疗效。将U87/EGFRvIII细胞皮下植入小鼠体内,然后用GW3965(每天口服40mg/kg)治疗12天。GW3965处理强烈诱导ABCA1表达并降低LDLR表达(图7A)。值得注意的是,这种活性伴随着59%的肿瘤生长抑制(图7B和C)和25倍的GBM细胞凋亡增加(图7D和E)。这些数据表明,LXR激动剂在体内能有效抑制GBM生长并促进肿瘤细胞死亡。 |
||
| 酶活实验 |
使用人LXRα的无细胞配体传感分析(LiSA)从葛兰素史克化合物文件的高通量筛选中鉴定出叔胺3。LXRαLiSA测量类固醇受体辅激活子1(SRC1)的24个氨基酸片段向受体配体结合结构域的配体依赖性募集[4]。
人洗血小板制剂[3] 人类血液是从自愿的健康志愿者身上采集的。将总共50 mL血液收集到含有3 mL 4%(重量/体积)抗凝剂柠檬酸钠的注射器中,并与7 mL酸性柠檬酸葡萄糖(ACD)混合,然后通过如前所述的差速离心制备洗涤后的血小板。35将血小板重新悬浮在改性的Tyrode-N-2-羟乙基哌嗪-N′-2-乙磺酸缓冲液(134mM NaCl、0.34mM Na2HPO4、2.9mM KCl、12mM NaHCO3、20mM N-2-羟乙基胡椒-N′-2-乙烯磺酸、5mM葡萄糖和1mM MgCl2,pH 7.3)中,并在实验前在30°C下静置30分钟。 血小板聚集和致密颗粒分泌测定[3] 血小板(4×108个细胞/mL)与荧光素在37°C下孵育2分钟,然后在光学光度计中用激动剂刺激,持续搅拌(1200 rpm)。如前所述,通过测量光密度的变化来确定血小板聚集35,通过使用荧光素萤光素酶系统试剂盒测量三磷酸腺苷(ATP)浓度的变化来测定致密颗粒分泌。 荧光光谱法测定[Ca2+]i[3] 如前所述,在预装有荧光染料Fluo-4NW的血小板中测量细胞内钙的动员。39用CRP或凝血酶刺激血小板,使用Fluoroskan上升板读数器在485nm处激发,在530nm处测量发射,测量钙的释放。 血小板内LXR-β的流式细胞术测量[3] 为了测量血小板内的LXR,将洗涤过的血小板用2%甲酰盐水固定,用BD Phosflow烫发缓冲液III渗透,洗涤,重新悬浮在HEPES缓冲液盐水中,并与LXR-β抗体一起孵育。然后洗涤血小板,将其重新悬浮在HBS中,与Cy3标记的第二抗体一起孵育,用0.2%甲酰盐水固定,并通过流式细胞术进行分析。使用适当的同种型对照设置阴性对照。 流式细胞术分析:α颗粒分泌和纤维蛋白原与整合素αIIbβ3的结合[3] 流式细胞术通过检测与αIIbβ3结合的纤维蛋白原水平和血小板表面P-选择素暴露水平,检测整合素αIIbα3和血小板α颗粒分泌的亲和力上调。用CRP刺激后,将血小板与异硫氰酸荧光素标记的纤维蛋白原和PE/Cy5抗人-CD62P(P-选择素)在室温下孵育20分钟。通过在0.2%(体积/体积)甲酰盐水中稀释100倍来停止反应。使用FACSCalibur设备进行流式细胞术采集,并使用CellQuest Pro软件版本3.3从5000个事件中收集数据。使用适当的IgG1κ-同种型匹配的对照来设置阴性对照,用于抗CD62P抗体和乙二醇四乙酸(10μM)用于纤维蛋白原结合。 体外血栓形成[3] 将柠檬酸全血与亲脂性染料3,3-二己氧基碳菁碘化物一起孵育,并以20 dyn/cm2的剪切速率通过胶原蛋白涂层(400μg/mL)Vena8Biochip灌注。使用尼康eclipse(TE2000-U)显微镜每30秒拍摄一次血栓Z图像,并使用幻灯片第5版计算血栓荧光强度。 |
||
| 细胞实验 |
将6×103个细胞接种到5%FBS的6孔板中24小时,然后换成1%LPDS培养基,用GW3965按时间顺序处理。用PBS洗涤细胞一次;然后根据其方案(Invitrogen)使用TRIzol试剂提取总RNA。接下来,将800ng RNA互补合成cDNA,并使用实时PCR(Bio-Rad)扩增,并将其值与每个复制的内部控制基因36B4(RPLP0)进行归一化。使用的引物如下:ABCA1正向:5′-ACAGTTGTGGCCCTTTG-3′,反向:5′-APGTCCAGGGCTGGGGTTC-3′;IDOL正向:5′-CGAGGCTGCCAACCA-3′,反向:5′-TGCAGGTCCAAAAGATACATCT-3′;36B4正向:5′-AATGCAGCATCTACAAC-CC-3′,反向:5′-TCGTTTTGTACCCGTTGATGA-3′[2]。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
In mice, 12/GW3965 showed 70% oral bioavailability with Cmax = 12.7 μg/mL and t1/2 = 2 h after dosing at 10 mg/kg. Analysis of the pharmacokinetic data indicated that the serum levels of 12/GW3965 were 5-fold above its EC50 in cells for up to 7 h after dosing. The pharmacological activity of 12/GW3965 was evaluated in C57BL/6 mice by dosing at 10 mg/kg bid for 14 days. By day 3, ABCA1 expression was increased 8-fold in the small intestine and 7-fold in peripheral macrophages (Figure 2A), while plasma levels of HDLc increased 30% at day 3 and was maintained until day 14 (Figure 2B). Thus, 12/GW3965 is an orally active LXR agonist that upregulates ABCA1 expression and raises circulating levels of HDL in C57BL/6 mice.[4]
|
||
| 参考文献 | |||
| 其他信息 |
Targeting LDLR with the liver X receptor (LXR) agonist GW3965 caused inducible degrader of LDLR (IDOL)–mediated LDLR degradation and increased expression of the ABCA1 cholesterol efflux transporter, potently promoting tumor cell death in an in vivo GBM model. These results show that EGFRvIII can promote tumor survival through PI3K/SREBP-1–dependent upregulation of LDLR and suggest a role for LXR agonists in the treatment of GBM patients.[2]
A potent, selective, orally active LXR agonist was identified from focused libraries of tertiary amines. GW3965 (12) recruits the steroid receptor coactivator 1 to human LXRalpha in a cell-free ligand-sensing assay with an EC(50) of 125 nM and profiles as a full agonist on hLXRalpha and hLXRbeta in cell-based reporter gene assays with EC(50)'s of 190 and 30 nM, respectively. After oral dosing at 10 mg/kg to C57BL/6 mice, 12 increased expression of the reverse cholesterol transporter ABCA1 in the small intestine and peripheral macrophages and increased the plasma concentrations of HDL cholesterol by 30%. 12 will be a valuable chemical tool to investigate the role of LXR in the regulation of reverse cholesterol transport and lipid metabolism.[4] Neuroactive steroid levels are decreased in the central nervous system (CNS) of streptozotocin (STZ) diabetic rats. In agreement, they exert protective effects in this experimental model, counteracting degenerative events occurring in the CNS. Therefore, an interesting therapeutic strategy could be to increase their levels directly in the CNS. In this study we have evaluated whether activation of translocator protein-18 kDa (TSPO) or liver X receptors (LXRs) may affect the levels of neuroactive steroids present in the CNS of diabetic and non-diabetic animals. We observed that the treatment with either Ro5-4864 (i.e., a ligand of TSPO) or with GW3965 (i.e., a ligand of LXRs) induced an increase of neuroactive steroids in the spinal cord, the cerebellum and the cerebral cortex of STZ-rats, but not in the CNS of non-pathological animals. Interestingly, the pattern of induction was different among the three CNS areas analyzed and between the two pharmacological tools. In particular, the activation of LXRs might represent a promising neuroprotective strategy, because the treatment with GW3965, at variance to Ro5-4864 treatment, did not induce significant changes in the plasma levels of neuroactive steroids. This suggests that activation of LXRs may selectively increase the CNS levels of neuroactive steroids avoiding possible endocrine side effects exerted by the systemic treatment with these molecules. Interestingly GW3965 treatment induced an increase of dihydroprogesterone in the spinal cord of diabetic animals in association with an increase of myelin basic protein expression. Thus we demonstrated that LXR activation was able to rescue CNS symptoms of diabetes. [1] Glioblastoma (GBM) is the most common malignant primary brain tumor of adults and one of the most lethal of all cancers. Epidermal growth factor receptor (EGFR) mutations (EGFRvIII) and phosphoinositide 3-kinase (PI3K) hyperactivation are common in GBM, promoting tumor growth and survival, including through sterol regulatory element-binding protein 1 (SREBP-1)–dependent lipogenesis. The role of cholesterol metabolism in GBM pathogenesis, its association with EGFR/PI3K signaling, and its potential therapeutic targetability are unknown. In our investigation, studies of GBM cell lines, xenograft models, and GBM clinical samples, including those from patients treated with the EGFR tyrosine kinase inhibitor lapatinib, uncovered an EGFRvIII-activated, PI3K/SREBP-1–dependent tumor survival pathway through the low-density lipoprotein receptor (LDLR). Targeting LDLR with the liver X receptor (LXR) agonist GW3965 caused inducible degrader of LDLR (IDOL)–mediated LDLR degradation and increased expression of the ABCA1 cholesterol efflux transporter, potently promoting tumor cell death in an in vivo GBM model. These results show that EGFRvIII can promote tumor survival through PI3K/SREBP-1–dependent upregulation of LDLR and suggest a role for LXR agonists in the treatment of GBM patients. [2] Liver X receptors (LXRs) are transcription factors involved in the regulation of cholesterol homeostasis. LXR ligands have athero-protective properties independent of their effects on cholesterol metabolism. Platelets are involved in the initiation of atherosclerosis and despite being anucleate express nuclear receptors. We hypothesized that the athero-protective effects of LXR ligands could be in part mediated through platelets and therefore explored the potential role of LXR in platelets. Our results show that LXR-β is present in human platelets and the LXR ligands, GW3965 and T0901317, modulated nongenomically platelet aggregation stimulated by a range of agonists. GW3965 caused LXR to associate with signaling components proximal to the collagen receptor, GPVI, suggesting a potential mechanism of LXR action in platelets that leads to diminished platelet responses. Activation of platelets at sites of atherosclerotic lesions results in thrombosis preceding myocardial infarction and stroke. Using an in vivo model of thrombosis in mice, we show that GW3965 has antithrombotic effects, reducing the size and the stability of thrombi. The athero-protective effects of GW3965, together with its novel antiplatelet/thrombotic [3] A potent, selective, orally active LXR agonist was identified from focused libraries of tertiary amines. GW3965 (12) recruits the steroid receptor coactivator 1 to human LXRalpha in a cell-free ligand-sensing assay with an EC(50) of 125 nM and profiles as a full agonist on hLXRalpha and hLXRbeta in cell-based reporter gene assays with EC(50)'s of 190 and 30 nM, respectively. After oral dosing at 10 mg/kg to C57BL/6 mice, 12 increased expression of the reverse cholesterol transporter ABCA1 in the small intestine and peripheral macrophages and increased the plasma concentrations of HDL cholesterol by 30%. 12 will be a valuable chemical tool to investigate the role of LXR in the regulation of reverse cholesterol transport and lipid metabolism. [4] |
| 分子式 |
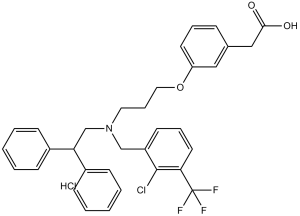
C33H31CLF3NO3.HCL
|
|
|---|---|---|
| 分子量 |
618.51
|
|
| 精确质量 |
617.171
|
|
| 元素分析 |
C, 64.08; H, 5.22; Cl, 11.46; F, 9.21; N, 2.26; O, 7.76
|
|
| CAS号 |
405911-17-3
|
|
| 相关CAS号 |
GW3965;405911-09-3
|
|
| PubChem CID |
16078973
|
|
| 外观&性状 |
Typically exists as white to off-white solids at room temperature
|
|
| LogP |
8.891
|
|
| tPSA |
49.77
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
13
|
|
| 重原子数目 |
42
|
|
| 分子复杂度/Complexity |
753
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
ClC1C(C(F)(F)F)=C([H])C([H])=C([H])C=1C([H])([H])N(C([H])([H])C([H])([H])C([H])([H])OC1=C([H])C([H])=C([H])C(C([H])([H])C(=O)O[H])=C1[H])C([H])([H])C([H])(C1C([H])=C([H])C([H])=C([H])C=1[H])C1C([H])=C([H])C([H])=C([H])C=1[H].Cl[H]
|
|
| InChi Key |
NMPUWJFHNOUNQU-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C33H31ClF3NO3.ClH/c34-32-27(15-8-17-30(32)33(35,36)37)22-38(18-9-19-41-28-16-7-10-24(20-28)21-31(39)40)23-29(25-11-3-1-4-12-25)26-13-5-2-6-14-26;/h1-8,10-17,20,29H,9,18-19,21-23H2,(H,39,40);1H
|
|
| 化学名 |
2-(3-(3-((2-chloro-3-(trifluoromethyl)benzyl)(2,2-diphenylethyl)amino)propoxy)phenyl)acetic acid hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.04 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (4.04 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.04 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 10 mg/mL (16.17 mM) in Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6168 mL | 8.0839 mL | 16.1679 mL | |
| 5 mM | 0.3234 mL | 1.6168 mL | 3.2336 mL | |
| 10 mM | 0.1617 mL | 0.8084 mL | 1.6168 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|---|
|