| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
BTK (IC50=0.5 nM)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
伊马替尼 (PCI-32765) 特异性抑制 B 细胞活性和信号传导。它可以防止 Btk (IC50=11 nM) 自身磷酸化、Btk 的生理底物 PLCγ (IC50=29 nM) 被磷酸化以及 ERK (IC50=13 nM)(更下游的激酶)被磷酸化[1]。伊马替尼 (PCI-32765) 抑制 BCR 激活的原代 B 细胞生长 (IC50=8 nM)。 Ibrutinib (PCI-32765) 在 FcγR 刺激后抑制原代单核细胞中 TNFα、IL-1β 和 IL-6 的产生(IC50 分别 = 2.6、0.5 和 3.9 nM)[3]。 Cysteine481 或 BTK 的 C481 与伊马替尼结合,最佳 IC50 为 0.5 nM。丝氨酸的羟基与伊马替尼不相容,C481S 突变将针对 BTK-C481S 磷酸化的 IC50 从 2.2 nM 提高至 1 μM[4]。
|
||
| 体内研究 (In Vivo) |
在患有胶原诱导关节炎的小鼠中,依鲁替尼 (PCI-32765)(3.125-50 mg/kg,口服)完全抑制疾病并降低循环自身抗体的量。在 MRL-Fas(lpr) 狼疮模型中,伊马替尼 (PCI-32765) 可防止自身抗体的形成和肾脏疾病的进展。在 MRL-Fas(lpr) 小鼠中,依鲁替尼 (PCI-32765)(3.125–50 mg/kg,口服)可改善肾脏疾病和自身抗体的产生[1]。与 T 细胞相比,依鲁替尼 (PCI-32765) (0.1 μM) 选择性地对 B 细胞产生细胞毒作用,但它会改变活化 T 细胞产生的细胞因子。当它被激活时,它还能抑制 CLL 细胞的增殖。在治疗性 CIA 模型中,依鲁替尼 (PCI-32765) 的 ED50 为 2.6 mg/kg/天,可有效且剂量依赖性地逆转关节炎炎症。依鲁替尼 (PCI-32765) 还可在 CAIA 模型中预防临床关节炎[3]。
|
||
| 酶活实验 |
将激酶, 33P-ATP, Ibrutinib, 和底物 [0.2 mg/mL 聚(EY)(4:1)]一起温育1小时后,使用33P 过滤结合实验测量体外激酶IC50值。
b细胞受体(BCR)信号在慢性淋巴细胞白血病(CLL)中异常激活。布鲁顿酪氨酸激酶(BTK)对BCR信号传导至关重要,在敲除小鼠模型中,其突变具有相对的B细胞特异性表型。本研究表明,与正常B细胞相比,BTK蛋白和mRNA在CLL中显著过表达。尽管BTK在CLL细胞中并不总是具有组成性活性,但BCR或CD40信号传导伴随着该途径的有效激活。使用不可逆的BTK抑制剂Ibrutinib (PCI-32765),我们发现CLL细胞的适度凋亡大于正常B细胞。未观察到Ibrutinib (PCI-32765)对t细胞存活的影响。用Ibrutinib (PCI-32765)处理CD40或BCR活化的CLL细胞可抑制BTK酪氨酸磷酸化,并有效地消除由该激酶激活的下游生存途径,包括ERK1/2、PI3K和NF-κB。此外,Ibrutinib (PCI-32765)在体外抑制活化诱导的CLL细胞增殖,并有效阻断微环境向CLL细胞提供的生存信号,包括可溶性因子(CD40L、BAFF、IL-6、IL-4和TNF-α)、纤连蛋白结合和基质细胞接触。基于这些集体数据,未来在CLL患者的临床试验中,有必要使用不可逆抑制剂Ibrutinib (PCI-32765)靶向BTK。[3] |
||
| 细胞实验 |
B和T细胞。CD20+B和CD3+T细胞通过阴性选择(RosetteSep,>90%纯度)从血沉棕黄层PBMC中纯化,并可在10%DMSO中冷冻。细胞在37°C下解冻,并保持在生长培养基(含有10%FCS的RPMI培养基)中。用山羊抗人IgM F(ab′)2(10μg/mL;Invitrogen)刺激B细胞,用1∶1珠/细胞比例的抗CD3/CD28包被珠(Dynabeads)刺激T细胞。用PE-CD69(BD Biosciences)对细胞进行染色,并通过流式细胞术对活淋巴细胞进行门控分析。尽管PCI-32765确实阻断了B细胞中抗IgM刺激的适度生存益处,但在实验过程中,浓度低于10μM的PCI-327650并没有降低B细胞或T细胞的生存能力。对于冲洗实验,在10体积的生长培养基中冲洗细胞三次,该方案被证实完全冲洗了可逆Btk抑制剂PCI-29732对BCR信号传导的抑制。[1]
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Ibrutinib is rapidly absorbed after oral administration and it presents a Cmax, tmax and AUC of approximately 35 ng/ml, 1-2 hour and 953 mg.h/ml respectively. The cumulative excretion of ibrutinib in urine is of about 7.8% of the administered dose and most of this excretion is found during the first 24 hours after administration. In feces, the cumulative excretion accounts for 80% of the administered dose and the excretion occurs within 48 hours of the initial administration. The total excretion of ibrutinib during the first 168 hours after initial administration accounts for 88.5% of the administered dose. The volume of distribution at steady-state of ibrutinib is in approximately 10,000 L. In patients with normal renal function, the clearance rate is in the range of 112-159 ml/min. Metabolism / Metabolites Three metabolic pathways have been identified according to the possible metabolites. These pathways are the hydroxylation of the phenyl group (M35), the opening of the piperidine with a reduction of the primary alcohol (M34) and the oxidation to a carboxylic acid and epoxidation of the ethylene followed by a hydrolysis to the formation of dihydrodiol (PCI-45227). The latter metabolite presents also 15 times lower inhibitory activity against BTK. The metabolism of ibrutinib is mainly performed by CYP3A5 and CYP3A4. and in a minor extent it is seen to be performed by CYP2D6. Since 2014, Ibrutinib has been available as a new drug for the treatment of leukemic diseases. Ibrutinib (Imbruvica) is metabolized in the liver mainly by the isoenzyme CYP3A4 and to a minor extent by CYP2D6. Simultaneous application of Imbruvica and consumption of foods containing secondary metabolites strongly inhibiting the CYP3A4 isoform, could significantly influence the toxicity of this drug. This article references the respective foods. Biological Half-Life The elimination half-life of ibrutinib is of approximately 4-6 hours. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Ibrutinib is an oral tyrosine kinase inhibitor that irreversibly binds and inhibits tyrosine-protein kinase BTK (Bruton tyrosine kinase). BTK is important in the function of B-cell receptor signaling and therefore in the maintenance and expansion of various B-cell malignancies. Targeting BTK with ibrutinib is an effective strategy in treating these malignancies. Ibrutinib, marketed as Imbruvica, is indicated for the treatment of patients with mantle cell lymphoma (MCL) or chronic lymphocytic leukemia (CLL) who have received at least one prior therapy. It is also indicated for the treatment of patients with chronic lymphocytic leukemia (CLL) with 17p deletion and patients with Waldenstrom's macroglobulinemia (WM). HUMAN EXPOSURE AND TOXICITY: Studies in humans have shown that ibrutinib may enhance chemoimmunotherapy efficacy without additive toxicities. Ibrutinib is cytotoxic to malignant plasma cells from patients with multiple myeloma (MM) and furthermore treatment with ibrutinib significantly augments the cytotoxic activity of bortezomib and lenalidomide chemotherapies. Hypersensitivity reactions including anaphylactic shock (fatal), urticaria, and angioedema have been reported. The outcome of patients with MCL who experience disease progression following ibrutinib therapy is poor, with both low response rates to salvage therapy and short duration of responses. ibrutinib inhibited the proliferation and induced apoptosis of Germinal center B-cell like diffuse large B-cell lymphoma (GCB-DLBCL) cell lines through suppression of BCR signaling pathway and activation of caspase-3. Furthermore, the chemokines CCL3 and CCL4 production from tumor cells were also found to be attenuated by ibrutinib treatment. Different cell lines exhibited distinct sensitivity after ibrutinib treatment. Interestingly, the decreasing level of p-ERK after ibrutinib treatment, but not the basal expression level of Btk, correlated with different drug sensitivity. Ibrutinib could be a potentially useful therapy for GCB-DLBCL and the decreasing level of p-ERK could become a useful biomarker to predict related therapeutic response. Ibrutinib is well tolerated when added to R-CHOP (chemotherapy named after the initials of the drugs used: rituximab, cyclophosphamide, doxorubicin (hydroxydaunomycin), vincristine (Oncovin ), prednisolone), and could improve responses in patients with B-cell non-Hodgkin lymphoma. ANIMAL STUDIES: Ibrutinib caused malformations in rats at exposures 14 times those reported in patients with MCL and 20 times those reported in patients with CLL or WM, receiving the ibrutinib dose of 560 mg per day and 420 mg per day, respectively. Reduced fetal weights were observed at lower exposures. Hepatotoxicity In the prelicensure clinical trials of ibrutinib in patients with CLL and mantle cell lymphoma, the rates of serum enzyme elevations during therapy were 20% to 30% but were similar to comparator arms, and elevations were generally mild (less than 5 times ULN) and self limited. In multiple controlled trials there were no reports of clinically apparent liver injury or need for early discontinuation because of hepatotoxicity. The major toxicities of ibrutinib resembled those of the tyrosine kinase receptor inhibitors and included hemorrhage and myelosuppression. While ibrutinib depressed peripheral lymphocyte counts and caused both lymphopenia and neutropenia, it has little effect on serum immunoglobulin levels and was not associated with reactivation of tuberculosis or opportunistic infections in prelicensure studies. Nevertheless, with approval and more widespread use of ibrutinib, rare cases of acute liver injury including acute liver failure and severe instances of reactivation of hepatitis B have been reported. The latency to onset of liver injury varied from several weeks to 9 months. The pattern of injury was hepatocellular, but the course was atypical of an acute hepatitis-like injury and more similar to acute hepatic necrosis with early onset of hepatic failure. Likelihood score: D (possible rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of ibrutinib during breastfeeding. Because ibrutinib is more than 97% bound to plasma proteins, the amount in milk is likely to be low. The manufacturer recommends that breastfeeding be discontinued during ibrutinib therapy and for 1 week after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Irreversible plasma protein binding increases gradually over time and reaches 25% of the administered dose 8 hours after initial administration. From the plasma proteins, ibrutinib has been shown to be mainly bound to albumin and to bind to α1 AGP. The irreversible protein binding of ibrutinib to plasma proteins can account for 97.3% of the administered dose. |
||
| 参考文献 |
|
||
| 其他信息 |
Therapeutic Uses
Ibrutinib is a novel oral tyrosine kinase inhibitor that irreversibly binds and inhibits tyrosine-protein kinase BTK (Bruton tyrosine kinase). BTK has been found to be important in the function of B-cell receptor signaling and therefore in the maintenance and expansion of various B-cell malignancies including chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL). Targeting BTK with ibrutinib has been found to be an effective strategy in treating these malignancies. Phase I clinical testing in non-Hodgkin's lymphomas and CLL showed that the drug was extremely well tolerated with no major dose-limiting toxicities and a 54% overall response rate. Subsequently, two phase Ib/II studies were performed on patients with CLL, one in relapsed/refractory CLL and one in previously untreated elderly patients with CLL. Both of these studies continued to show good tolerability of the drug and an overall response rate of about 71% with extended duration of response. Another phase II study using ibrutinib in relapsed/refractory MCL was conducted and also showed that it was well tolerated with an overall response rate of 68% and extended duration of response. Due to these results, the U.S. Food and Drug Administration granted accelerated approval for ibrutinib in November 2013 for patients with MCL who had received at least one prior therapy and in February 2014 for patients with CLL who had received at least one prior therapy. This review will discuss the preclinical pharmacology, pharmacokinetics and clinical efficacy to date of ibrutinib in the treatment of CLL and MCL. /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health(NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Ibrutinib is included in the database. Imbruvica is indicated for the treatment of patients with mantle cell lymphoma (MCL) who have received at least one prior therapy. Accelerated approval was granted for this indication based on overall response rate. Continued approval for this indication may be contingent upon verification of clinical benefit in confirmatory trials. /Included in US product label/ Imbruvica is indicated for the treatment of patients with chronic lymphocytic leukemia (CLL) who have received at least one prior therapy. /Included in US product label/ For more Therapeutic Uses (Complete) data for IBRUTINIB (7 total), please visit the HSDB record page. Drug Warnings Fatal and non-fatal infections have occurred with IMBRUVICA therapy. Grade 3 or greater infections occurred in 14% to 26% of patients. Cases of progressive multifocal leukoencephalopathy (PML) have occurred in patients treated with IMBRUVICA. Monitor patients for fever and infections and evaluate promptly. Fatal bleeding events have occurred in patients treated with IMBRUVICA. Grade 3 or higher bleeding events (subdural hematoma, gastrointestinal bleeding, hematuria and post procedural hemorrhage) have occurred in up to 6% of patients. Bleeding events of any grade, including bruising and petechiae, occurred in approximately half of patients treated with IMBRUVICA. The mechanism for the bleeding events is not well understood. IMBRUVICA may increase the risk of hemorrhage in patients receiving antiplatelet or anticoagulant therapies. Consider the benefit-risk of withholding IMBRUVICA for at least 3 to 7 days pre and post-surgery depending upon the type of surgery and the risk of bleeding. Treatment-emergent Grade 3 or 4 cytopenias including neutropenia (range, 19 to 29%), thrombocytopenia (range, 5 to 17%), and anemia (range, 0 to 9%) occurred in patients treated with IMBRUVICA. Monitor complete blood counts monthly. Atrial fibrillation and atrial flutter (range, 6 to 9%) have occurred in patients treated with IMBRUVICA, particularly in patients with cardiac risk factors, acute infections, and a previous history of atrial fibrillation. Periodically monitor patients clinically for atrial fibrillation. Patients who develop arrhythmic symptoms (e.g., palpitations, lightheadedness) or new onset dyspnea should have an ECG performed. If atrial fibrillation persists, consider the risks and benefits of IMBRUVICA treatment and dose modification. For more Drug Warnings (Complete) data for IBRUTINIB (8 total), please visit the HSDB record page. Pharmacodynamics In vitro studies have shown an induction of CLL cell apoptosis even in presence of prosurvival factors. It has also been reported an inhibition of CLL cell survival and proliferation as well as an impaired in cell migration and a reduction in the secretion of chemokines such as CCL3 and CCL4. The latter effect has been shown to produce regression in xenograft mouse models. Clinical studies for relapsed/refractory CLL in phase I and II showed an approximate 71% of overall response rate.. In the case of relapsed/refractory mantle cell lymphoma, approximately 70% of the tested patients presented a partial or complete response.. In clinical trials for relapsed/refractory diffuse large B-cell lymphoma, a partial response was found in between 15-20% of the patients studied; while for patients with relapsed/refractory Waldenstrom's macroglobulinemia, a partial response was observed in over 75% of the patients tested. Finally, for patients with relapsed/refractory follicular lymphoma, a partial to complete response was obtained in approximately 54% of the patients. |
| 分子式 |
C25H24N6O2
|
|---|---|
| 分子量 |
440.5
|
| 精确质量 |
440.196
|
| 元素分析 |
C, 68.17; H, 5.49; N, 19.08; O, 7.26
|
| CAS号 |
936563-96-1
|
| 相关CAS号 |
Ibrutinib Racemate;936563-87-0;Ibrutinib-d5;1553977-17-5
|
| PubChem CID |
24821094
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
715.0±60.0 °C at 760 mmHg
|
| 熔点 |
149-158ºC
|
| 闪点 |
386.2±32.9 °C
|
| 蒸汽压 |
0.0±2.3 mmHg at 25°C
|
| 折射率 |
1.696
|
| LogP |
2.92
|
| tPSA |
99.16
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
678
|
| 定义原子立体中心数目 |
1
|
| SMILES |
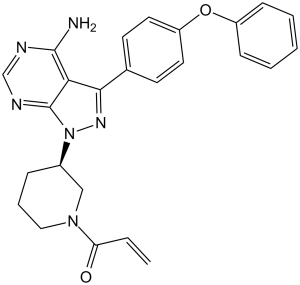
NC1N=CN=C2N([C@@H]3CCCN(C(=O)C=C)C3)N=C(C=12)C1C=CC(OC2C=CC=CC=2)=CC=1
|
| InChi Key |
XYFPWWZEPKGCCK-GOSISDBHSA-N
|
| InChi Code |
InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1
|
| 化学名 |
(R)-1-(3-(4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidin-1-yl)prop-2-en-1-one.
|
| 别名 |
PCI-32765; PCI-32765, Ibrutinib, PCI 32765, trade name: Imbruvica
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.75 mg/mL (6.24 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.68 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: 2.5 mg/mL (5.68 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 配方 4 中的溶解度: ≥ 2.5 mg/mL (5.68 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 配方 5 中的溶解度: Formulation 1: ~10 mg/mL (23 mM) in 2% DMSO+Castor oil, 澄清溶液 Formulation 2: ≥ 2.5 mg/mL (5.7 mM) in 5% DMSO + 95% (20% SBE-β-CD in saline), 澄清溶液 Formulation 3: ≥ 2.5 mg/mL (5.7 mM) in 10% DMSO + 90% (20% SBE-β-CD in saline), 悬浮液 Formulation 4: ≥ 2.5 mg/mL (5.7 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + + 45% Saline, 澄清溶液 Formulation 5: ≥ 2.5 mg/mL (5.7 mM) in 10% DMSO + 90% Corn oil, 澄清溶液 Formulation 6: ~3.4 mg/mL (7.6 mM) in 0.5% MC+ 0.5% Tween-80, 悬浮液 配方 6 中的溶解度: 3.33 mg/mL (7.56 mM) in 0.5% MC 0.5% Tween-80 (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2701 mL | 11.3507 mL | 22.7015 mL | |
| 5 mM | 0.4540 mL | 2.2701 mL | 4.5403 mL | |
| 10 mM | 0.2270 mL | 1.1351 mL | 2.2701 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Testing the Combination of Venetoclax and Rituximab, in Comparison to the Usual Treatment (Ibrutinib and Rituximab) for Waldenstrom's Macroglobulinemia/Lymphoplasmacytic Lymphoma
CTID: NCT04840602
Phase: Phase 2 Status: Recruiting
Date: 2024-11-26
|
|
|