| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
SGLT; hSGLT2 (IC50 = 7.4 nM); hSGLT1 (IC50 = 1876 nM); rSGLT2 (IC50 = 6.73 nM); rSGLT1 (IC50 = 1166 nM); mSGLT2 (IC50 = 5.64 nM); mSGLT1 (IC50 = 1380 nM)
|
|---|---|
| 体外研究 (In Vitro) |
Ipragliflozin (1-50 μM) 以剂量依赖性方式显着抑制人乳腺癌细胞系 MCF-7 的增殖。 Ipragliflozin 最初会降低乳腺癌细胞增殖,但当使用 siRNA 敲低 SGLT2 表达时,这种效应被完全消除。这表明 Ipragliflozin 抑制 SGLT2 以最大限度地减少乳腺癌细胞增殖。 BrdU 测定表明,高剂量(50 和 100 μM)的 Ipragliflozin 会显着降低 MCF-7 细胞中的 DNA 产量 [1]。
SGLT2和SGLT1抑制试验[1] Ipragliflozin浓度依赖性抑制小鼠、大鼠和人在纳摩尔浓度下的SGLT2活性(表1)。ipragliflozin对人SGLT2的抑制作用约为phlorizin的5倍,但对人SGLT1的抑制作用仅为phlorizin的1 / 9。根皮素对两种SGLT的抑制作用都很小。ipragliflozin、phlorizin和phloretin的选择性比(human SGLT1/SGLT2的IC50)分别为254、6和3。此外,ipragliflozin对小鼠和大鼠SGLT2具有高选择性。达格列净也能有效地、选择性地抑制小鼠和人SGLT2活性。 特异性测定[1] 为了确认特异性,使用放射性配体结合和酶分析检测了Ipragliflozin对几种代表性受体、通道和转运体的影响。伊普列净不与多种受体、离子通道和转运体相互作用,如肾上腺素能(α1、α2和β)、毒碱碱(M1、M2和非选择性)、血管紧张素(AT1和AT2)、钙通道(l型和n型)、钾通道(KATP和SKCa)、钠通道(2号位点)、胆囊收缩素(CCKA和CCKB)、多巴胺(D1、D2和转运体)、内皮素(ETA和ETB)、γ -氨基丁酸(GABAA和GABAB)、谷氨酸(AMPA、kainate和NMDA)、血清素(5-HT1、5HT2B、组胺(H1、H2和H3)和神经激肽(NK1、NK2和NK3), IC50值为0 ~ 3000 nM。 Ipragliflozin对小鼠肠道葡萄糖苷酶的稳定性[1] 采用小鼠小肠粘膜匀浆法测定了异格列净和苯连菌素对葡萄糖苷酶的体外生物稳定性。虽然ipragliflozin完全没有被降解(图2a),但在小鼠粘膜匀浆中,根连素被迅速降解为其糖基,根连素(Fig. 2b)。 癌症是目前2型糖尿病患者死亡的主要原因之一。我们之前报道了胰高血糖素样肽-1受体激动剂exendin-4对前列腺癌和乳腺癌的有益作用。在本研究中,我们通过乳腺癌模型检测了钠-葡萄糖共转运蛋白2 (SGLT2)抑制剂Ipragliflozin的抗癌作用。在人乳腺癌MCF-7细胞中,采用RT-PCR和免疫组织化学检测SGLT2的表达。Ipragliflozin在1 ~ 50 μM浓度下显著且剂量依赖性地抑制MCF-7细胞的生长。BrdU实验还显示,ipragliflozin以剂量依赖的方式减弱MCF-7细胞的增殖。因为ipragliflozin对乳腺癌细胞的作用通过敲除SGLT2而被完全取消,所以Ipragliflozin可以通过抑制SGLT2来起作用。接下来,我们使用膜片钳技术测量了膜电位和全细胞电流。用ipragliflozin或无糖培养基处理MCF-7细胞时,观察到膜超极化。此外,无葡萄糖培养基和siRNA敲除SGLT2抑制了葡萄糖诱导的MCF-7细胞的全细胞电流,表明ipragliflozin抑制了钠和葡萄糖通过SGLT2的共转运。ipragliflozin显著增加了JC-1绿色荧光,提示线粒体膜电位发生了变化。这些发现表明,SGLT2抑制剂ipragliflozin通过膜超极化和线粒体膜不稳定来减弱乳腺癌细胞的增殖。[2] |
| 体内研究 (In Vivo) |
Ipragliflozin 具有降血糖特性。血糖水平的升高受到伊格列净 (0.1-1 mg/kg) 剂量依赖性的抑制。这种效应在 STZ 诱导的 1 型糖尿病大鼠中在 0.3 和 1 mg/kg 剂量下显着,在 KK-Ay 2 型糖尿病小鼠中在所有测试剂量下均显着[1]。在链脲佐菌素诱导的 1 型糖尿病大鼠中,重复剂量的伊格列净(0.3 和 1 mg/kg)显示出抗糖尿病作用 [1]。
对新型SGLT2选择性抑制剂伊格列净(Ipragliflozin, ASP1941; (1S)-1,5-脱水-1-C-{3-[(1-苯并噻吩-2-基)甲基]-4-氟苯基}-D: -葡萄糖醇与L: -脯氨酸(1:1)的化合物)的药理学特征进行了研究。在体外,评估了伊格列净抑制SGLT2和SGLT1的效力以及稳定性。在体内,在正常小鼠、链脲佐菌素诱导的1型糖尿病大鼠和KK-Ay 2型糖尿病小鼠中研究了伊格列净的药代动力学和药理学特征。伊格列净在纳摩尔范围内有效且选择性地抑制人、大鼠和小鼠的SGLT2,并表现出对肠道葡萄糖苷酶的稳定性。口服给药后,伊格列净显示出良好的药代动力学特性,并剂量依赖性地增加尿葡萄糖排泄,该效应在正常小鼠中持续超过12小时。单次给药伊格列净在两种糖尿病模型中均产生剂量依赖性和持续的抗高血糖作用。此外,在两种糖尿病模型中,每日一次伊格列净治疗4周改善了高血糖,同时伴随着尿葡萄糖排泄的增加。相比之下,在药理学剂量下,伊格列净不影响正常血糖(如格列本脲的情况),并且不影响肠道葡萄糖吸收和电解质平衡。这些结果表明,伊格列净是一种口服有效的SGLT2选择性抑制剂,它通过抑制肾脏葡萄糖重吸收来诱导尿葡萄糖排泄持续增加,随后产生抗高血糖作用且低血糖风险低。因此,伊格列净通过增加葡萄糖向尿液中的排泄,具有治疗糖尿病高血糖的治疗潜力。[1] 伊格列净对正常小鼠尿葡萄糖排泄的影响 [1] 在正常小鼠中,Ipragliflozin/伊格列净(0.01–10 mg/kg)剂量依赖性地显著增加尿葡萄糖排泄,并且在剂量≥0.3 mg/kg时,该效应在给药后12–18小时仍然明显(图4a)。在3和10 mg/kg剂量下,尿量也显著增加(图4b)。 单次给药伊格列净对糖尿病动物的影响[1] 在STZ诱导的1型糖尿病大鼠和KK-Ay 2型糖尿病小鼠中,伊格列净(0.1−1 mg/kg)剂量依赖性地降低血糖水平,并且该效应在所有测试剂量下均显著(图5a和图6a)。在给药后12小时进行口服葡萄糖耐量试验(OGTT)期间,伊格列净(0.1–1 mg/kg)剂量依赖性地抑制血糖水平的升高。在STZ诱导的1型糖尿病大鼠中,该效应在0.3和1 mg/kg剂量下显著(图5b),而在KK-Ay 2型糖尿病小鼠中,该效应在所有测试剂量下均显著(图6b)。 重复给药伊格列净对STZ诱导的1型糖尿病大鼠的影响 [1] 与正常对照组大鼠相比,在非禁食条件下,STZ诱导的1型糖尿病大鼠的HbA1c、血糖和尿葡萄糖排泄的平均水平显著更高,血浆胰岛素水平和胰腺胰岛素含量显著更低(表2)。重复给药伊格列净(0.3和1 mg/kg)4周显著降低了HbA1c和血糖水平。血浆胰岛素水平未发生显著变化,但胰腺胰岛素含量在1 mg/kg剂量下显著增加。尿葡萄糖排泄呈剂量依赖性增加,并且在1 mg/kg剂量下显著增加。在整个研究期间,伊格列净不影响体重或食物摄入量(数据未显示)。 重复给药伊格列净对KK-Ay 2型糖尿病小鼠的影响 [1] 重复给药伊格列净(0.3和1 mg/kg)4周降低了HbA1c和血糖水平,同时伴随着尿葡萄糖排泄的增加(表3)。此外,尿白蛋白排泄显著减少。在整个研究期间,伊格列净治疗不影响体重或食物摄入量(数据未显示)。 伊格列净对正常小鼠空腹血糖水平的影响 [1] 在正常小鼠中,伊格列净(0.03–100 mg/kg)剂量依赖性地抑制葡萄糖负荷后血糖水平的升高,并且该效应在剂量≥0.1 mg/kg时显著(图7a)。格列本脲(0.3–300 mg/kg)也剂量依赖性地抑制血糖水平的升高;该效应在剂量≥3 mg/kg时显著(图7c)。在过夜禁食的小鼠中,伊格列净(0.03–100 mg/kg)剂量依赖性地降低血糖水平,但该效应仅在剂量≥10 mg/kg(比OGTT中的剂量高100倍)时才显著(图7b)。格列本脲(0.3–300 mg/kg)在与OGTT相同的剂量下也剂量依赖性地降低空腹血糖水平(图7d)。在空腹条件下,伊格列净不改变血浆胰岛素水平,但显著降低了葡萄糖负荷条件下血浆胰岛素水平的升高。相比之下,格列本脲在两种条件下均显著增加血浆胰岛素水平(数据未显示)。 伊格列净对正常小鼠胃肠道碳水化合物含量的影响 [1] 在正常小鼠中进行液体膳食负荷后,胃肠道二糖(蔗糖和麦芽糖)和单糖(葡萄糖和果糖)含量显著增加(图8)。伏格列波糖(1 mg/kg)显著增加胃肠道二糖含量(图8a, b),降低单糖含量(图8c, d),并显著抑制血糖水平的升高(数据未显示)。相比之下,伊格列净(0.3–30 mg/kg)即使在最高剂量下也未显著影响胃肠道二糖含量。此外,伊格列净未显著影响胃肠道果糖含量,虽然它确实剂量依赖性地增加了葡萄糖含量,但该效应仅在30 mg/kg的最大剂量时才显著。伊格列净剂量依赖性地抑制血糖水平的升高,并且该效应在所有测试剂量下均显著(数据未显示)。 伊格列净对KK-Ay 2型糖尿病小鼠血浆和尿液参数的影响 [1] 在2型糖尿病小鼠中,袢利尿剂呋塞米(10 mg/kg)显著增加尿电解质(Na+, K+, 和 Cl−)排泄和尿量,同时伴随着尿渗透压的降低(表4)。这种由电解质排泄引起的显著利尿作用还导致血浆电解质浓度显著降低和血浆渗透压显著升高。加压素V1A/V2受体拮抗剂YM471(3 mg/kg)在不增加电解质排泄的情况下显著增加尿量,并显著降低尿渗透压。这种显著的利尿作用导致血浆电解质浓度和渗透压显著升高。相比之下,伊格列净(1 mg/kg)显著增加尿葡萄糖排泄,同时伴随着尿量的轻微增加,并显著降低血糖水平,但不显著影响血浆或尿电解质平衡。 |
| 酶活实验 |
SGLT2和SGLT1抑制试验[1]
克隆了人类、大鼠和小鼠SGLT2和SGLT1全长互补脱氧核糖核酸序列,并使用先前描述的标准技术稳定地转染到中国仓鼠卵巢(CHO)细胞中(Katsuno et al. 2007)。细胞接种于含10%胎牛血清的Ham’s F12培养基中,以3 × 104个/孔的密度接种于96孔板中。细胞在镀后1天使用。测试化合物首先溶解在二甲亚砜中,然后用钠缓冲液(140 mM NaCl, 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM n -2-羟乙基哌嗪- n ' -2-乙磺酸,5 mM Tris-HCl, pH 7.4)稀释到所需浓度。取出培养基后,细胞在100μl preincubated胆碱分析缓冲区(氯化钠在测定钠缓冲替换相同浓度的氯化胆碱)在37°C 20分钟。然后他们被孵化的测试化合物的解决方案(25μl)包含14 c-amg(2.2μCi /毫升)和nonlabeled AMG(最终浓度55μM) 37°C 2 h。200μl细胞被洗两次冰冷的清洗缓冲(胆碱分析缓冲区包含10毫米AMG),然后随着0.5%十二烷基硫酸钠(SDS)解决方案(25μl)。将细胞裂解液与75 μl MicroScint MS-40混合,用Top Count微孔板闪烁计数器测定放射性。 |
| 细胞实验 |
细胞活力测定[2]
细胞类型: MCF-7 人乳腺癌细胞系 测试浓度: 1、10、50 μM <孵育持续时间:24、48、72、96小时 实验结果:以剂量依赖性方式减少MCF-7细胞的数量。 细胞培养和细胞增殖试验[2] MCF-7和MDA-MB-231人乳腺癌细胞系购自美国类型培养库。所有细胞在添加10%胎牛血清(FBS)和1%青霉素/链霉素的Dulbecco 's Modified Eagle 's Medium (DMEM)中维持。细胞增殖实验按照前面所述进行,并进行了轻微修改。简单地说,将细胞置于12孔组织培养板中,并在0-50 μM Ipragliflozin的完整培养基中维持。0-4天后,用血细胞计进行细胞计数分析细胞增殖情况。 溴脱氧尿苷(BrdU)测定[2] 使用细胞增殖ELISA试剂盒进行BrdU掺入试验。简单地说,将MCF-7细胞以5000个/孔的速度在96孔培养皿中完全培养液中进行培养。达到60%-70%的一致性后,细胞用含有10% FBS (0-100 μM Ipragliflozin)的培养基处理24小时。在刺激的最后2小时加入BrdU溶液(10 μM)。将细胞干燥并固定,用FixDenat溶液在室温下去除细胞DNA 30分钟。在培养板中加入过氧化物酶偶联的小鼠抗brdu单克隆抗体,室温孵育90 min。加入四甲基联苯胺底物,在室温下孵育15分钟。样品的吸光度用酶标仪在450 - 620 nm处测定。平均数据表示为相对于对照(未处理)细胞增殖的比率。 SGLT2小干扰(si)RNA敲低及细胞增殖试验[2] 为了敲除SGLT2,我们使用了SGLT-2 siRNA,这是针对人类SGLT2设计的;siRNA作为阴性对照。将MCF-7细胞以2 × 105个/孔的比例在六孔板中进行细胞层析,使用MISSION siRNA转染试剂转染10 nmol/L SGLT-2 siRNA或阴性对照siRNA。转染72小时后,进行细胞增殖试验。简单地说,将细胞分离并重新镀于24孔组织培养板中,在含或不含10 μM Ipragliflozin的完整培养基中。在治疗后0-4天,收集细胞并用血细胞计计数。 线粒体通透性电位[2] 根据公司的说明书,使用JC-1线粒体膜电位检测试剂盒检测线粒体膜电位(ΔΨm)。MCF-7细胞经10 μM Ipragliflozin处理或不处理后,用阳离子染料JC-1染色,在线粒体中表现出电位依赖性积累。在低膜电位下,JC-1作为单体存在并产生绿色荧光(527 nm发射)。在高膜电位和极化下,JC-1形成J聚集体并产生红色荧光(发射波长为590nm)。 |
| 动物实验 |
Animal/Disease Models: Single Administration[1] Streptozotocin (STZ; 50 mg/kg)-induced type 1 diabetic rats and KK-Ay type 2 diabetic mice
Doses: 0.1-1 mg/kg Route of Administration: Single oral administration in the fed condition. Blood glucose levels were then measured for 8 h under fasting conditions. Experimental Results: Dose-dependently lowered blood glucose levels, and this effect was significant at all tested doses. Animal/Disease Models: Repeated Administration[1] Streptozotocin (STZ; 50 mg/kg)- induced type 1 diabetic rats Doses: 0.3 and 1 mg/kg Route of Administration: Administration orally one time/day (at night) for 4 weeks. Experimental Results: Dramatically decreased the levels of HbA1c and blood glucose. Pancreatic insulin content was Dramatically increased at a dose of 1 mg/kg. Urinary glucose excretion was increased dose-dependently, and this was significant at the 1 mg/kg dose.\ Stability against mouse intestinal glucosidases [1] Under ether anesthesia, the small intestine was removed from overnight fasted normal mice, washed with cold saline, excised, and rinsed with phosphate buffer (48 mM NaCl, 5.4 mM KCl, 28 mM Na2HPO4, 43 mM NaH2PO4, 35 mM mannitol, 10 mM glucose, pH 6.5). The mucosal tissue was scraped off gently using a slide glass, homogenized with phosphate buffer, and used for the stability study. Test compounds were initially dissolved in acetonitrile at a concentration of 5 mM and then diluted to 100 μM with the phosphate buffer. Mucosal homogenates (5 mg/ml, 100 μl) were preincubated at 37°C in microtubes. Thereafter, each compound solution (100 μl, final concentration 50 μM) was added and incubated at 37°C for varying time periods. The reaction was stopped by the addition of ice-cold acetonitrile (200 μl), then 200 μl of methyl tert-butyl ether was added, and the mixture was centrifuged (15,000 rpm, 10 min). The supernatant was transferred into a tube and evaporated in a vacuum centrifugal concentrator. The residue was dissolved in mobile phase for use as the assay sample. Concentrations of compound in the assay sample were analyzed using a high-performance liquid chromatography (HPLC) with an ultraviolet detector (265 nm for Ipragliflozin and 280 nm for phlorizin and phloretin) and a 4.6 × 250-mm reversed-phase ODS-80Ts column. The column temperature was maintained at 60°C, 20 mM ammonium acetate/acetonitrile [20:80 (v/v)] was used as the mobile phase, and the flow rate was 1.5 ml/min. Pharmacokinetics [1] After oral administration of ipragliflozin (3 mg/kg) or phlorizin (100 mg/kg) to non-fasted normal mice, blood was withdrawn from the abdominal vena cava under ether anesthesia. The plasma concentrations of Ipragliflozin or phlorizin were measured using HPLC. Acetonitrile (100 μl) and methyl tert-butyl ether (100 μl) were added to the plasma samples (100 μl), mixed, and then centrifuged (15,000 rpm, 10 min). The supernatant was transferred into a tube and evaporated in a vacuum centrifugal concentrator. The residue was dissolved in HPLC mobile phase, 0.1% formic acid solution/acetonitrile [55:45 (v/v)] for use as the assay sample. Concentrations of ipragliflozin or phlorizin in the assay samples were measured as described above. Effect of Ipragliflozinon urinary glucose excretion in normal mice [1] Ipragliflozin (0.01–10 mg/kg) was administered to non-fasted normal mice, and spontaneously voided urine was collected for 24 h after administration while the animals were kept in metabolic cages. After the urine volume had been measured, the glucose concentration in the urine was measured using the Glucose CII test reagent. Effect of single administration of Ipragliflozin in diabetic animals [1] To investigate its antihyperglycemic effect, ipragliflozin (0.1–1 mg/kg) was administered to STZ-induced type 1 diabetic rats and KK-Ay type 2 diabetic mice in the fed condition. Blood glucose levels were then measured for 8 h under fasting conditions, in order to eliminate the influence of feeding during the experiment. To evaluate sustainability, ipragliflozin (0.1–1 mg/kg) was administered to both types of diabetic animals, which were then fasted for 12 h (overnight). A glucose solution (2 g/kg) was subsequently administered orally, and blood glucose levels were measured as described above. Effect of repeated administration of Ipragliflozin in STZ-induced type 1 diabetic rats [1] Ipragliflozin (0.3 and 1 mg/kg) was administered to STZ-induced type 1 diabetic rats once daily (at night) for 4 weeks. Body weight and food intake were measured every week. After drug administration on day 26, rats were transferred to metabolic cages and spontaneously voided urine was collected for 24 h. On the morning after the final drug administration on day 28, blood samples were collected under non-fasting conditions, and the pancreas was isolated under ether anesthesia. Blood and urinary glucose concentrations were measured as described above. The pancreas was homogenized by adding acid–ethanol solution (75% ethanol, 23.5% purified water, and 1.5% concentrated hydrochloric acid) and incubating at 4°C for 1 h to extract the insulin. Subsequently, the culture was centrifuged and the supernatant was used as a measurement sample. Plasma and pancreatic insulin concentrations were measured using an enzyme-linked immunosorbent assay (ELISA) kit. Hemoglobin A1c (HbA1c) levels were measured using a DCA2000 System. Effect of repeated administration of Ipragliflozin in KK-Ay type 2 diabetic mice [1] Ipragliflozin (0.3 and 1 mg/kg) was administered to KK-Ay type 2 diabetic mice once daily (at night) for 4 weeks. Body weight and food intake were measured every week. On the morning after the drug administration on day 28, blood samples were collected under non-fasting conditions. After the drug administration on day 30, mice were transferred to metabolic cages and spontaneously voided urine was collected for 24 h. Blood and urinary glucose concentrations, HbA1c, and plasma insulin levels were measured as described above. Urinary albumin concentration was measured using a mouse albumin ELISA. Effect of Ipragliflozin on fasting blood glucose levels in normal mice [1] In order to investigate their effects on postprandial hyperglycemia, ipragliflozin (0.03–100 mg/kg) or glibenclamide (0.3–300 mg/kg) was administered to normal mice that had been fasted overnight. After 30 min, glucose solution (2 g/kg) was administered orally, and blood glucose levels were measured. To investigate their effects on hypoglycemia, ipragliflozin (0.03–100 mg/kg) or glibenclamide (0.3–300 mg/kg) was administered to normal mice fasted overnight, and blood glucose levels were measured. Effect of Ipragliflozin on gastrointestinal carbohydrate contents in normal mice [1] After fasting for 24 h, mice received ipragliflozin (0.3–30 mg/kg) or voglibose (1 mg/kg). After 15 min, a liquid meal (ENSURE·H: carbohydrates 206 mg/ml, fats 53 mg/ml, proteins 53 mg/ml) was given orally at 20 ml/kg. Control mice were given purified water instead of a liquid meal. At 1 h after the liquid meal or water administration, blood glucose levels were measured, and gastrointestinal tracts (stomach, upper and lower small intestine, cecum, and lower large intestine) were isolated under ether anesthesia. Isolated gastrointestinal tracts were homogenized with purified water (5 ml) and centrifuged (3,000 rpm, 10 min) to retrieve the supernatant. Glucose concentration was measured as described above. Sucrose and maltose concentrations were measured by a previously described method with minor modifications (Dörner 1977). Fructose concentration was measured using a fructose assay kit. Effect of Ipragliflozin on plasma and urinary parameters in KK-Ay type 2 diabetic mice [1] Ipragliflozin (1 mg/kg), furosemide (10 mg/kg), or YM471 (3 mg/kg) was administered to non-fasted diabetic mice, which were then transferred to metabolic cages. The mice were allowed free access to food and water, and spontaneously voided urine samples were collected for 8 h. Thereafter, blood samples were collected from the tail vein for the determination of blood glucose level. Under ether anesthesia, urine was collected from the bladder, and blood samples were collected from the abdominal vena cava. The volume of spontaneously voided urine combined with the urine in the bladder was measured. Blood and urine samples were centrifuged (15,000 rpm, 10 min), after which the supernatants were used for the determination of several parameters. Plasma and urine osmolalities were measured using a freezing point depression osmometer. Plasma and urine electrolyte (Na+, K+, and Cl−) concentrations were determined using a flame photometer, and the urinary electrolyte excretion was calculated as the product of the urine electrolyte concentration and the urine volume. |
| 药代性质 (ADME/PK) |
Pharmacokinetics [1]
After oral administration of Ipragliflozin (3 mg/kg) to normal mice, plasma concentrations of ipragliflozin reached a maximum at 1 h and then gradually decreased (Fig. 3). Obvious plasma concentrations were detected even 8 h after administration. In contrast, when phlorizin (100 mg/kg) was administered orally, plasma drug concentrations were low and rapidly eliminated. |
| 参考文献 | |
| 其他信息 |
Ipragliflozin is a glycoside.
Ipragliflozin is under investigation in Type 2 Diabetes and Diabetes Mellitus, Type 2. SGLT2, which is specifically expressed in the kidneys, plays an important role in renal glucose reabsorption (Jabbour and Goldstein 2008). In contrast, SGLT1 is highly expressed in the small intestine and mediates dietary glucose absorption (Pajor and Wright 1992). In order to elucidate the in vivo effect of ipragliflozin against intestinal SGLT1, we examined its effect on gastrointestinal carbohydrate absorption. α-Glucosidase inhibitors, such as voglibose, inhibit the small intestinal disaccharidases, sucrase and maltase (Matsuo et al. 1992). This slows the absorption of carbohydrates from the small intestine, thereby lowering postprandial hyperglycemia (Baron 1998). In this study, voglibose induced a significant increase in intestinal disaccharide content (by delaying disaccharide digestion) that inhibited the increase in blood glucose levels. Thus, α-glucosidase inhibitors are effective at preventing postprandial hyperglycemia by this mechanism. However, osmotic water retention induced by accumulation of intestinal disaccharide content can cause gastrointestinal symptoms such as soft feces or diarrhea (Vichayanrat et al. 2002). Ipragliflozin, however, did not affect intestinal disaccharide or fructose content, but at the maximum dose of 30 mg/kg, it did significantly increase intestinal glucose content. It is known that gastrointestinal expression of SGLTs and absorption of glucose depend mainly on SGLT1 (Turk et al. 1991) and that gastrointestinal drug concentrations immediately after oral administration are at a very high level (Masaoka et al. 2006). Thus, although ipragliflozin shows a 245-fold higher selectivity for mouse SGLT2 versus SGLT1, the significant increase in gastrointestinal glucose content with the highest dose of ipragliflozin is thought to be due to the inhibition of glucose absorption via SGLT1 in the small intestine. Nevertheless, gastrointestinal glucose elevation was only significant at a dose which is 100 times higher than that which significantly decreased postprandial hyperglycemia. It is therefore considered that therapeutic doses of ipragliflozin would not inhibit intestinal SGLT1, nor affect intestinal carbohydrate digestion and absorption. As such, the risk of gastrointestinal symptoms should be low. During the course of both acute and chronic experiments, no gastrointestinal side effects indicative of significant intestinal SGLT1 inhibition were noted at any dose. Higher doses of Ipragliflozin slightly increased urine volume along with a significant increase in urinary glucose excretion. Since sodium-ion transportation accompanies the glucose transport promoted by SGLT2, we evaluated the effect of Ipragliflozin on plasma and urinary electrolyte balance. In this experiment, the loop diuretic, furosemide, induced a marked diuresis with concomitant increase in urinary electrolyte excretion, and decreased plasma concentrations of electrolytes including sodium. This furosemide-induced hyponatremia may be a severe adverse reaction (Sonnenblick et al. 1993). The vasopressin V1A/V2 receptor antagonist, YM471, also exerted a potent aquaretic effect and increased plasma electrolyte concentrations. In contrast, ipragliflozin (1 mg/kg) markedly increased urinary glucose excretion with a concomitant slight increase in urine volume, but did not affect plasma or urinary electrolyte balance. These results suggest that ipragliflozin would increase urinary glucose excretion without inducing either a marked osmotic diuresis or an electrolyte imbalance at pharmacological doses. Mutations in the SGLT2 gene lead to the rare disorder, familial renal glucosuria, where glucose reabsorption in the kidneys is severely impaired and large amounts of glucose are excreted in the urine (Francis et al. 2004; Magen et al. 2005). Despite the severe glucosuria, patients appear to have a benign condition, with no serious adverse events or problems in kidney function. Familial renal glucosuria is also not associated with hypoglycemia and generally has no significant clinical manifestations (Santer et al. 2003). In this study, chronic administration of pharmacological doses of ipragliflozin did not induce significant adverse effects in diabetic models. Based on these findings, Ipragliflozin seems to be an effective and safe drug for the treatment of hyperglycemia. In conclusion, the present study shows that Ipragliflozin is a potent selective SGLT2 inhibitor, possesses good pharmacokinetic characteristics, and exhibits a sustained antihyperglycemic effect by increasing urinary glucose excretion, without inducing hypoglycemia or insulin secretion. These results indicate the potential usefulness of ipragliflozin for further development as a therapeutic agent for hyperglycemia in patients with diabetes. Clinical trials with ipragliflozin are currently in progress, and proof-of-concept studies should clarify the suitability of ipragliflozin for the treatment of diabetes (Kashiwagi et al. 2010). [1] ion in human breast cancer cells, which is not expressed in human normal mammary gland. Our findings revealed that the SGLT2 inhibitor Ipragliflozin attenuated breast cancer cell proliferation and DNA synthesis (Fig. 1). The dose of ipragliflozin that attenuated breast cancer cell proliferation, 1–10 μM, was similar to its pharmacologi‐ cal concentration in serum, suggesting that our data generally mirror clinical conditions. Furthermore, orally administrated ipragliflozin distributes into glandular tis‐ sues in similar or higher concentrations compared with serum (unpublished data by Astellas Pharma). Although growth was also suppressed at a lower dose of ipragliflo‐ zin (Fig. 2A), ipragliflozin at a high dose, 50–100 μM, reduced DNA synthesis in the BrdU assay (Fig. 2C). These findings suggest that ipragliflozin attenuated breast cancer cell proliferation through not only inhibit‐ ing DNA synthesis but also other mechanisms, such as cell death including apoptosis. We examined apoptosis by TUNEL assay, however, reproducible apoptosis was not observed. Further examination using other methods is required. We focused on the sodium transport by SGLT2 because sodium uptake is emerging as a mech‐ anism of cancer biology including breast cancer. Ipragliflozin shut down sodium uptake through SGLT2 and induced membrane hyperpolarization of MCF-7 cells. We also found that ipragliflozin induced instability of ΔΨm, which may lead to apoptosis and necrosis of host cells. The mechanism by which SGLT2 induces mitochondrial membrane instability may involve either inhibition of glucose or inhibition of sodium transport. Intracellular sodium could alter ΔΨm via sodiumcalcium and sodium-hydrogen exchangers. However, low glucose might not regulate ΔΨm; glucose transporter 1 is also expressed in breast cancer cells and is an important energy regulator and therapeutic target. Further experiments may reveal other effects of SGLT2 inhibitors on cancer cells and explore combina‐ tion treatment with SGLT2 inhibitor, metformin and GLP-1. A meta-analysis and case report suggested anticancer effects of SGLT2 inhibitors. In conclusion, in this study, we showed that the SGLT2 inhibitor Ipragliflozin attenuates breast cancer cell proliferation via membrane hyperpolarization and mitochondrial membrane instability [2] |
| 分子式 |
C21H21FOS
|
|
|---|---|---|
| 分子量 |
404.45
|
|
| 精确质量 |
404.109
|
|
| 元素分析 |
C, 62.36; H, 5.23; F, 4.70; O, 19.78; S, 7.93
|
|
| CAS号 |
761423-87-4
|
|
| 相关CAS号 |
Ipragliflozin (L-Proline);951382-34-6
|
|
| PubChem CID |
10453870
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.5±0.1 g/cm3
|
|
| 沸点 |
628.8±55.0 °C at 760 mmHg
|
|
| 闪点 |
334.1±31.5 °C
|
|
| 蒸汽压 |
0.0±1.9 mmHg at 25°C
|
|
| 折射率 |
1.684
|
|
| LogP |
5.59
|
|
| tPSA |
118.39
|
|
| 氢键供体(HBD)数目 |
4
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
4
|
|
| 重原子数目 |
28
|
|
| 分子复杂度/Complexity |
525
|
|
| 定义原子立体中心数目 |
5
|
|
| SMILES |
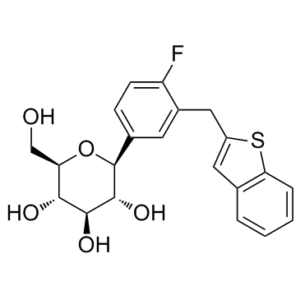
FC1=CC=C([C@H]2[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O2)C=C1CC3=CC(C=CC=C4)=C4S3
|
|
| InChi Key |
AHFWIQIYAXSLBA-RQXATKFSSA-N
|
|
| InChi Code |
InChI=1S/C21H21FO5S/c22-15-6-5-12(21-20(26)19(25)18(24)16(10-23)27-21)7-13(15)9-14-8-11-3-1-2-4-17(11)28-14/h1-8,16,18-21,23-26H,9-10H2/t16-,18-,19+,20-,21+/m1/s1
|
|
| 化学名 |
(2S,3R,4R,5S,6R)-2-[3-(1-benzothiophen-2-ylmethyl)-4-fluorophenyl]-6-(hydroxymethyl)oxane-3,4,5-triol
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.18 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.18 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.18 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4725 mL | 12.3625 mL | 24.7249 mL | |
| 5 mM | 0.4945 mL | 2.4725 mL | 4.9450 mL | |
| 10 mM | 0.2472 mL | 1.2362 mL | 2.4725 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Non-inferiority of iPragliflozin and metformin on glucose metabolism, pleiotropic effects and safety in type2 diabetes
CTID: UMIN000018979
Phase: Status: Recruiting
Date: 2015-09-11
Stability ofaipragliflozin andbphlorizin in mouse intestinal mucosal homogenates.Naunyn Schmiedebergs Arch Pharmacol.2012 Apr;385(4):423-36. |
|---|
Effects of ipragliflozin onaurinary glucose excretion andburine volume in normal mice.Naunyn Schmiedebergs Arch Pharmacol.2012 Apr;385(4):423-36. |
Effects of ipragliflozin on blood glucose levels in streptozotocin-induced type 1 diabetic rats.Naunyn Schmiedebergs Arch Pharmacol.2012 Apr;385(4):423-36. |
Effects of ipragliflozin on blood glucose levels in KK-Aytype 2 diabetic mice.Naunyn Schmiedebergs Arch Pharmacol.2012 Apr;385(4):423-36. |
|---|
Effects of ipragliflozin and glibenclamide on fasting blood glucose levels in normal mice.Naunyn Schmiedebergs Arch Pharmacol.2012 Apr;385(4):423-36. |