| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
HIV-1 (WT)(EC50=0.068 nM);HIV-1 (MDR)(EC50=0.15 nM);HIV-1 (M184V)(EC50=3.1 nM)
|
|---|---|
| 体外研究 (In Vitro) |
Islatravir (MK-8591) (4'Ed2FA) 是一种强效抗 HIV-1 药物,对于 HIV-1 (WT)、HIV-1 (M184V) 和 HIV 的 EC50 分别为 0.068 nM、3.1 nM 和 0.15 nM分别为-1(MDR)。它作为核苷逆转录酶抑制剂发挥作用[1]。
|
| 体内研究 (In Vivo) |
Islatravir(EFdA)治疗导致大多数接受治疗的小鼠在治疗后3周内PB中的HIV-RNA降至无法检测的水平。EFdA治疗的BLT小鼠颈阴道灌洗液中的HIV-RNA水平也降至检测不到的水平,表明EFdA强烈渗透到FRT中。我们的研究结果还表明,在所有分析的组织中,HIV复制都受到了强烈的系统性抑制。特别是,我们观察到,与未经治疗的HIV感染对照小鼠相比,EFdA治疗的BLT小鼠的胃肠道和FRT中的HIV-RNA水平存在2倍以上的差异。此外,与未经治疗的HIV感染对照小鼠相比,EFdA治疗的BLT小鼠的淋巴结、肝脏、肺、脾脏中的HIV-RNA也显著降低。此外,EFdA治疗防止了PB、粘膜组织和淋巴组织中CD4+T细胞的耗竭[2]
|
| 酶活实验 |
基于一个工作假设,提出了使用4'-C-取代-2'-脱氧核苷衍生物的想法,以解决现有获得性免疫缺陷综合征化疗(高效抗逆转录病毒疗法)的问题。随后的研究成功地证明了这一想法的有效性,并开发了2'-脱氧-4'-C-乙炔基-2-氟腺苷,这是一种核苷逆转录酶抑制剂,对所有人类免疫缺陷病毒1型(HIV-1s)都非常有效,包括多药耐药性HIV-1,并且毒性低[1]。
|
| 细胞实验 |
标本采集和处理[2]
在HIV暴露前后6周内纵向(每周)收集PB和CVL样本。将PB收集在EDTA中,通过在300g下离心5分钟分离血浆用于HIV-RNA分析。用PBS重构剩余的血细胞以恢复PB样品的原始体积,并用于流式细胞术分析。通过用无菌PBS进行宫颈阴道灌洗(CVL,第0-5周)获得宫颈阴道分泌物(CVS)(每次洗涤3次,每次20μl,总体积约60μl)。为确保手术无创,使用20μl无菌过滤吸管头进行CVL,吸管头插入阴道腔不超过1-3mm。离心(300g 5分钟)后,使用无细胞上清液进行HIV-RNA分析。将颗粒重新悬浮在PBS中,用于流式细胞术分析。在HIV暴露后6周的尸检中采集骨髓(BM)、LN、人胸腺类器官(ORG)、肝脏、肺、脾脏、胃肠道(从十二指肠到直肠)和FRT(阴道、宫颈和子宫),并如前所述分离单核细胞进行HIV-RNA、HIV-DNA和流式细胞术分析。 HIV病毒载量和流式细胞术分析[2] 使用一步逆转录酶实时PCR[ABI定制TaqMan设计检测法(检测限(LOD):血浆-750拷贝/ml,CVL-1400拷贝/60μl)测量PB和CVL HIV-RNA水平。低于检测限的血浆和CVL病毒载量水平分别绘制为375拷贝/ml和700拷贝/ml。我们使用这些值计算各组的平均值。通过实时RT-PCR(HIV-RNA,LOD-1.5拷贝/105细胞和HIV-DNA,LOD为2.5拷贝/105电池)确定从组织分离的单核细胞中HIV-RNA和HIV-DNA的存在。作为从人类细胞中提取的DNA存在的对照。,所有样本均通过实时PCR检测人类γ珠蛋白DNA的存在。 |
| 动物实验 |
Virus challenge and administration of EFdA[2]
Stocks of HIV-1JR-CSF were prepared via transient transfection of 293 T cells, and titred using TZM-bl cells as previously described. HIV-1JR-CSF (30,000 TCIU) was administered intravenously by tail vein injection. EFdA was reconstituted in phosphate-buffered saline (PBS) at a concentration of 1 mg/mL and administered orally to BLT mice by oral gavage at 10 mg/kg once daily for 3 weeks beginning at 3 weeks post-HIV infection. PBS (200 μL) was administered by oral gavage to (untreated) controls. |
| 参考文献 |
|
| 其他信息 |
Islatravir is an investigational drug that is being studied to treat and prevent HIV infection.
Islatravir belongs to a group of HIV drugs called nucleosidereverse transcriptase translocation inhibitors (NRTTIs). NRTTIs use several different methods to block an HIV enzyme called reverse transcriptase. By blocking reverse transcriptase, NRTTIs prevent HIV from multiplying and can reduce the amount of HIV in the body. Islatravir may be effective against certain HIV strains that are resistant to other HIV drugs. Islatravir is under investigation in clinical trial NCT04233216 (Doravirine/islatravir (DOR/ISL) in Heavily Treatment-experienced (HTE) Participants for Human Immunodeficiency Virus Type 1 (HIV-1) Infection (MK-8591A-019)). Drug Indication Prevention of human immunodeficiency virus (HIV-1) infection. An idea to use 4'-C-substituted-2'-deoxynucleoside derivatives was proposed based on a working hypothesis to solve the problems of existing acquired immune deficiency syndrome chemotherapy (highly active antiretroviral therapy). Subsequent studies have successfully proved the validity of the idea and resulted in the development of 2'-deoxy-4'-C-ethynyl-2-fluoroadenosine, a nucleoside reverse transcriptase inhibitor, which is highly potent to all human immunodeficiency viruses type 1 (HIV-1s) including multidrug-resistant HIV-1 and has a low toxicity.[1] Background: The nucleoside reverse transcriptase inhibitor (NRTI) 4'-ethynyl-2-fluoro-2'-deoxyadenosine (EFdA) in preclinical development exhibits improved safety and antiviral activity profiles with minimal drug resistance compared to approved NRTIs. However, the systemic antiviral efficacy of EFdA has not been fully evaluated. In this study, we utilized bone marrow/liver/thymus (BLT) humanized mice to investigate the systemic effect of EFdA treatment on HIV replication and CD4+ T cell depletion in the peripheral blood (PB) and tissues. In particular, we performed a comprehensive analysis of the female reproductive tract (FRT) and gastrointestinal (GI) tract, major sites of transmission, viral replication, and CD4+ T cell depletion and where some current antiretroviral drugs have a sub-optimal effect. Results: EFdA treatment resulted in reduction of HIV-RNA in PB to undetectable levels in the majority of treated mice by 3 weeks post-treatment. HIV-RNA levels in cervicovaginal lavage of EFdA-treated BLT mice also declined to undetectable levels demonstrating strong penetration of EFdA into the FRT. Our results also demonstrate a strong systemic suppression of HIV replication in all tissues analyzed. In particular, we observed more than a 2-log difference in HIV-RNA levels in the GI tract and FRT of EFdA-treated BLT mice compared to untreated HIV-infected control mice. In addition, HIV-RNA was also significantly lower in the lymph nodes, liver, lung, spleen of EFdA-treated BLT mice compared to untreated HIV-infected control mice. Furthermore, EFdA treatment prevented the depletion of CD4+ T cells in the PB, mucosal tissues and lymphoid tissues. Conclusion: Our findings indicate that EFdA is highly effective in controlling viral replication and preserving CD4+ T cells in particular with high efficiency in the GI and FRT tract. Thus, EFdA represents a strong potential candidate for further development as a part of antiretroviral therapy regimens.[2] |
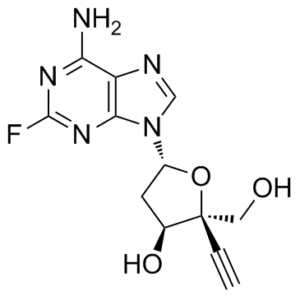
| 分子式 |
C12H12FN5O3
|
|---|---|
| 分子量 |
293.253785133362
|
| 精确质量 |
293.09
|
| 元素分析 |
C, 49.15; H, 4.12; F, 6.48; N, 23.88; O, 16.37
|
| CAS号 |
865363-93-5
|
| 相关CAS号 |
EFdA-TP;950913-56-1; 2408129-39-3 (hydrate)
|
| PubChem CID |
6483431
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
-0.6
|
| tPSA |
119
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
21
|
| 分子复杂度/Complexity |
459
|
| 定义原子立体中心数目 |
3
|
| SMILES |
C#C[C@]1([C@H](C[C@@H](O1)N2C=NC3=C(N=C(N=C32)F)N)O)CO
|
| InChi Key |
IKKXOSBHLYMWAE-QRPMWFLTSA-N
|
| InChi Code |
InChI=1S/C12H12FN5O3/c1-2-12(4-19)6(20)3-7(21-12)18-5-15-8-9(14)16-11(13)17-10(8)18/h1,5-7,19-20H,3-4H2,(H2,14,16,17)/t6-,7+,12+/m0/s1
|
| 化学名 |
(2R,3S,5R)-5-(6-amino-2-fluoro-9H-purin-9-yl)-2-ethynyl-2-(hydroxymethyl)tetrahydrofuran-3-ol
|
| 别名 |
MK-8591; 4′-ethynyl-2-fluoro-2′-deoxyadenosine; EFdA; Islatravir; 865363-93-5; 4'-Ethynyl-2-Fluoro-2'-Deoxyadenosine; MK-8591; Islatravir [USAN]; (2R,3S,5R)-5-(6-amino-2-fluoro-9H-purin-9-yl)-2-ethynyl-2-(hydroxymethyl)tetrahydrofuran-3-ol; ISLATRAVIR ANHYDROUS; ISL; MK8591; MK 8591
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~341.01 mM )
H2O : ~3.57 mg/mL (~12.17 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (7.09 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (7.09 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (7.09 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 1.1 mg/mL (3.75 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 5 中的溶解度: ≥ 1.1 mg/mL (3.75 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 6 中的溶解度: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.08 mg/mL (7.09 mM) 配方 7 中的溶解度: 1.35 mg/mL (4.60 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4101 mL | 17.0503 mL | 34.1006 mL | |
| 5 mM | 0.6820 mL | 3.4101 mL | 6.8201 mL | |
| 10 mM | 0.3410 mL | 1.7050 mL | 3.4101 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。