| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 500mg |
|
||
| 1g | |||
| Other Sizes |
| 体外研究 (In Vitro) |
研究结果表明,isoxsuprine salticide 以剂量依赖性方式(5 至 60 μM)抑制 12(S)-HETE 合成以及循环化学排斥剂诱导缺陷 (CCID) 的产生。此外,只有盐酸异索素才能抑制另外两种迁移指标 MYPT、桩蛋白和 MLC2 的诱导 [2]。
|
|---|---|
| 体内研究 (In Vivo) |
用载体治疗的动物的总梗塞体积为 279 ± 25 mm3,而用异氧磺酸盐治疗的动物的总梗塞体积为 137 ± 18 mm3 [3]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Isoxsuprine hydrochloride is almost completely absorbed from the gastrointestinal tract. After oral admin of the drug, peak plasma concns occur within 1 hr and persist for about 3 hr. ...Isoxsurprine crosses the placenta. The drug is partially conjugated in the body and is excreted in the urine. Fecal excretion of the drug is negligible. Biological Half-Life Mean plasma half-life of the drug is 1.25 hr. |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
Concurrent heavy use /of tobacco smoking/ may interfere with the therapeutic effects of isoxsuprine because nicotine constricts blood vessels. Non-Human Toxicity Values LD50 Rat oral 1750 mg/kg LD50 Rat intraperitoneal 164 mg/kg |
| 参考文献 |
|
| 其他信息 |
Isoxsuprine hydrochloride is an alkylbenzene.
Isoxsuprine Hydrochloride is the hydrochloride salt of isoxsuprine, a benzyl alcohol derivative with vasodilator activity. The mechanism of action of isoxsuprine hydrochloride is controversial because this drug has beta-adrenergic agonist activities that are not reversed by beta-adrenergic blockers. Although stimulation of the beta adrenergic receptor increases blood flow to produce vasodilatation, this drug may also have direct effects on the contractility of smooth muscle. Isoxsuprine hydrochloride also causes relaxation of uterine smooth muscle and may also produce positive inotropic and chronotropic effects on the myocardium. A beta-adrenergic agonist that causes direct relaxation of uterine and vascular smooth muscle. Its vasodilating actions are greater on the arteries supplying skeletal muscle than on those supplying skin. It is used in the treatment of peripheral vascular disease and in premature labor. See also: Isoxsuprine (has active moiety). Mechanism of Action Isoxsuprine produces peripheral vasodilation by a direct effect on vascular smooth muscle, primarily within skeletal muscle with little effect on cutaneous blood flow. Its effects were once thought to be due to beta-adrenergic receptor stimulation but are not reversed by beta-adrenergic blocking agents. Therapeutic Uses Adrenergic beta-Agonists; Sympathomimetics; Tocolytic Agents; Vasodilator Agents Isoxsuprine is also used for management of threatened premature labor in pregnancies of 20 or more weeks' gestation. Use in not recommended prior to the 20th week of pregnancy. For isoxsuprine to be most effective, it is recommended that therapy be started as soon as the diagnosis of preterm labor is confirmed. Efficacy in advanced labor has not been established. Use in patients with ruptured membranes must be weighed against the risk of intrauterine infection. /NOT included in US product labeling/ Isoxsuprine has been used in the treatment of dysmenorrhea. /NOT included in US product labeling/ FDA has classified isoxsuprine as being possibly effective for its labeled indications, which include relief of symptoms associated with cerebrovascular insufficiency and peripheral vascular disease, ie, arteriosclerosis obliterans, thromboangiitis obliterans (Buerger's disease), and Raynaud's disease. This classification requires the submission of adequate and well-controlled studies in order to provide substantial evidence of effectiveness. /Included in US product labeling/ For more Therapeutic Uses (Complete) data for ISOXSUPRINE HYDROCHLORIDE (7 total), please visit the HSDB record page. Drug Warnings Except under special circumstances, this medication /isoxsuprine hydrochloride/ should not be used immediately postpartum or when the following medical problems exist: For use in management of premature labor only: Cardiac disorders, especially those associated with arrhythmias, or maternal hyperthyroidism (isoxsuprine may precipitate arrhythmias or heart failure; occult cardiac disease may be unmasked) or chorioamnionitis (intrauterine infection) or hemorrhage or intrauterine fetal death or known abnormality (immediate delivery required) or eclampsia (toxemia) and severe pre-eclampsia or pulmonary hypertension. Isoxsuprine has both beta-1 and beta-2 adrenergic activity. Maternal hypotension and tachycardia are common side effects. Hypocalcemia, hypoglycemia, hypotension, ileus, and neonatal death are increased after isoxsuprine administration. ...When injected sc in human beings causes several mm of mercury transient elevation of ocular pressure, max in an hr or two, associated with edema and hyperemia of episcleral tissues. Adverse effects of isoxsuprine include trembling, nervousness, weakness, dizziness, flushing, transient palpitation, tachycardia, chest pain, hypotension, abdominal distress, nausea, vomiting, intestinal distention, and severe rash. For more Drug Warnings (Complete) data for ISOXSUPRINE HYDROCHLORIDE (8 total), please visit the HSDB record page. |
| 分子式 |
C18H23NO3.HCL
|
|---|---|
| 分子量 |
337.84
|
| 精确质量 |
337.144
|
| CAS号 |
579-56-6
|
| 相关CAS号 |
Isoxsuprine-d6 hydrochloride;2706004-35-3
|
| PubChem CID |
11368
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.146g/cm3
|
| 沸点 |
484.2ºC at 760mmHg
|
| 熔点 |
203-204°
|
| 闪点 |
246.6ºC
|
| LogP |
4.064
|
| tPSA |
61.72
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
23
|
| 分子复杂度/Complexity |
299
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
QVPSGVSNYPRFAS-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C18H23NO3.ClH/c1-13(12-22-17-6-4-3-5-7-17)19-14(2)18(21)15-8-10-16(20)11-9-15;/h3-11,13-14,18-21H,12H2,1-2H3;1H
|
| 化学名 |
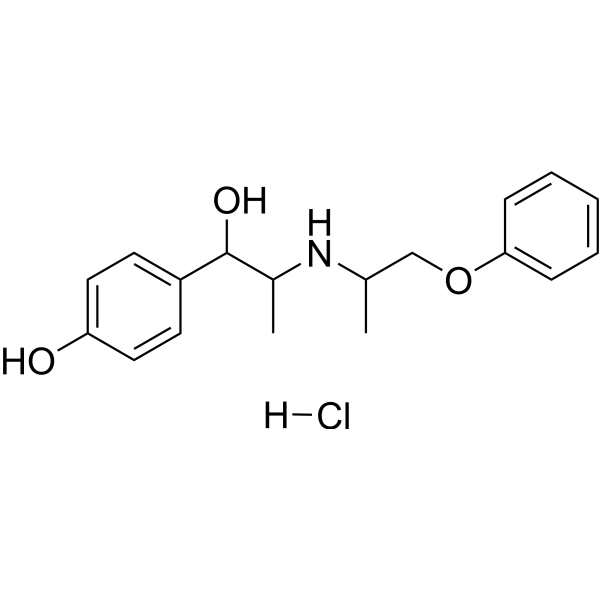
4-[1-hydroxy-2-(1-phenoxypropan-2-ylamino)propyl]phenol;hydrochloride
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~140 mg/mL (~414.40 mM)
H2O : ~15.56 mg/mL (~46.06 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 3.5 mg/mL (10.36 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 35.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 3.5 mg/mL (10.36 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 35.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 3.5 mg/mL (10.36 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9600 mL | 14.7999 mL | 29.5998 mL | |
| 5 mM | 0.5920 mL | 2.9600 mL | 5.9200 mL | |
| 10 mM | 0.2960 mL | 1.4800 mL | 2.9600 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。