| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
BET/bromodomain and extra terminal domain
Bromodomain and Extra-Terminal (BET) Family Proteins (BRD4 Bromodomain 1: Ki=2.3 nM for (+)-JQ1 carboxylic acid binding; BRD4 Bromodomain 2: Ki=3.1 nM) [1] |
|---|---|
| 体外研究 (In Vitro) |
在 B16F10 细胞表面,JQ-1 羧酸可降低 PD-L1 的表达 [1]。
(+)-JQ1羧酸((+)-JQ1 carboxylic acid) 是一种BET溴结构域靶向配体,作为三价PROTAC的靶向弹头用于特异性BET蛋白降解[1] - BET蛋白结合选择性:与BRD4溴结构域1(BD1)和溴结构域2(BD2)结合,Ki值分别为2.3 nM和3.1 nM;对非BET溴结构域(如BRD7、BRD9)的选择性>100倍,Ki>500 nM[1] - 支持PROTAC介导的BET降解:通过其羧基官能团与三价PROTAC偶联后,(+)-JQ1 carboxylic acid 保留BET结合亲和力,使PROTAC在MV4;11白血病细胞中诱导剂量依赖性BRD4降解(PROTAC的DC₅₀=120 nM,western blot)[1] - 无 (+)-JQ1 carboxylic acid 单独的抗增殖活性数据;含该配体的三价PROTAC在72小时MTT实验中抑制MV4;11细胞增殖,IC₅₀=350 nM[1] |
| 体内研究 (In Vivo) |
(+)-JQ1 (50 mg/kg) 抑制 NMC 797 异种移植小鼠的肿瘤生长。 (+)-JQ1 (50 mg/kg) 导致 NMC 797 异种移植小鼠中 NUT 核斑点的消失,这与与核染色质的竞争性结合一致。 (+)-JQ1 (50 mg/kg) 在 NMC 797 异种移植物中诱导强(31 级)角蛋白表达。 (+)-JQ1 (50 mg/kg) 促进 NMC 异种移植小鼠模型的分化、肿瘤消退和延长生存期。与媒介物处理的动物相比,静脉注射 MM.1S-luc+ 细胞后,(+)-JQ1 (50 mg/kg) 导致原位异种移植的 SCID 米色小鼠的总生存期显着延长。 (+)-JQ1 (50 mg/kg ip) 导致带有 Raji 异种移植物的小鼠的存活率显着增加。
|
| 酶活实验 |
BET溴结构域结合实验(AlphaScreen法):重组人BRD4 BD1/BD2结构域用实验缓冲液(50 mM HEPES pH 7.5、100 mM NaCl、0.01% Tween-20、1 mM DTT)稀释。将 (+)-JQ1 carboxylic acid 系列3倍稀释液(0.1 nM–1 μM)与BRD4结构域、生物素化乙酰化组蛋白H4肽(底物)在384孔板中混合,加入链霉亲和素偶联供体珠和抗GST受体珠(针对GST标签化BRD4),室温孵育1小时。检测AlphaScreen信号,非线性回归分析计算Ki值[1]
|
| 细胞实验 |
PROTAC介导的BRD4降解实验:MV4;11白血病细胞以2×10⁶个/孔接种到6孔板,用含 (+)-JQ1 carboxylic acid 的三价PROTAC(0.01–1 μM)处理24小时。RIPA缓冲液裂解细胞,SDS-PAGE分离蛋白,膜与抗BRD4和GAPDH(内参)一抗孵育,HRP偶联二抗检测,密度分析法量化条带强度以确定BRD4降解效率[1]
- 细胞增殖实验(MTT):MV4;11细胞以5×10³个/孔接种到96孔板,用含 (+)-JQ1 carboxylic acid 的三价PROTAC(0.01–5 μM)处理72小时。加入MTT试剂37°C孵育4小时,测定570 nm处吸光度,计算PROTAC的IC₅₀值(无 (+)-JQ1 carboxylic acid 单独数据)[1] |
| 动物实验 |
In vivo formulations used (reported):
1. Dissolved in 5% dextrose; 50 mg/kg; i.p. injection; Nature. 2010 Dec 23;468(7327):1067-73 2. Dissolved in 10% DMSO and 90% of a 10% 2-hydroxypropyl-β-cyclodextrin solution; Leukemia. 2017 Oct;31(10):2037-2047 3. Dissolved in 1% DMSO+5% Glucose+ddH2O; Cell. 2018 Sep 20;175(1):186-199.e19 4. Dissolved in 20% hydroxypropyl-β-cyclodextrin, 5% DMSO, 0.2% Tween-80 in saline; Mol Cancer Ther. 2016 Jun;15(6):1217-26 5. Dissolved in 1:1 propylene glycol:water; J Biol Chem. 2016 Nov 4;291(45):23756-23768 6. Dissolved in 5% DMSO in 10% 2-hydroxypropyl-β-cyclodextrin solution; Cancer Lett. 2017 Aug 28;402:100-109 |
| 参考文献 | |
| 其他信息 |
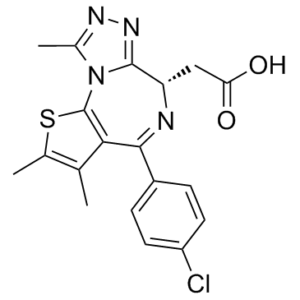
Trivalent PROTACs having a functionalization site with controlled orientation were designed, synthesized, and evaluated. Based on the X-ray structure of BRD protein degrader MZ1 (1) in complex with human VHL and BRD4BD2, we expected that the 1,2-disubstituted ethyl group near the JQ-1 moiety in MZ1 (1) could be replaced by a planar benzene tether as a platform for further functionalization. To test this hypothesis, we first designed six divalent MZ1 derivatives, 2a-c and 3a-c, by combining three variations of substitution patterns on the benzene ring (1,2-, 1,3-, and 1,4-substitution) and two variations in the number of ethylene glycol units (2 or 1). We then tested the synthesized compounds for the BRD4 degradation activity of each. As expected, we found that 1,2D-EG2-MZ1 (2a), an MZ1 derivative with 1,2-disubstituted benzene possessing two ethylene glycol units, had an activity profile similar to that of MZ1 (1). Based on the structure of 2a, we then synthesized and evaluated four isomeric trivalent MZ1 derivatives, 15a-15d, having a tert-butyl ester unit on the benzene ring as a handle for further functionalization. Among the four isomers, 1,2,5T-EG2-MZ1 (15c) retained a level of BRD4 depletion activity similar to that of 2a without inducing a measurable Hook effect, and its BRD4 depletion kinetics was the same as that of MZ1 (1). Other isomers were also shown to retain BRD4 depletion activity. Thus, the trivalent PROTACs we synthesized here may serve as efficient platforms for further applications.[1]
Intratumoral environment as a hypoxic, non-inflamed "cold" state is difficult for many agents to accumulate and activate the immune system. Intrinsically, facultative anaerobic Salmonella VNP20009 target the tumor hypoxic areas, invade into tumor cells and exhibit an immune effect. Here we engineer the bacteria by decorating their surface with newly synthesized heptamethine cyanine dyes NHS-N782 and JQ-1 derivatives to obtain the biohybrid agent N-V-J, leading to the deep tumor targeted photothermal therapy and magnified immunotherapy. Due to the mitochondrial targeting capacity of NHS-N782, N-V-J becomes susceptive to the temperature rise when reaching tumors. This synergistic strategy promotes the systemic immunity by creating an inflamed "hot" tumor state from three different dimensions, which include the inherent immunogenicity of bacteria, the near-infrared laser triggered tumor antigens and the downregulation of PD-L1 expression. All these approaches result in effective and long-lasting T cell immune responses to prevent local and distant tumors for extended time. Leveraging the attenuated bacteria to transport dual drugs to the tumor tissues for self-synthetic vaccines provides a novel paradigm to enhance the bacteria-mediated cancer immunotherapy.[2] (+)-JQ1 carboxylic acid is a carboxyl-functionalized derivative of (+)-JQ1, a potent BET bromodomain inhibitor, designed as a targeting warhead for PROTAC synthesis [1] - Structural feature: The carboxylic acid group (-COOH) serves as a conjugation site to link with PROTAC linkers and effector moieties, enabling the formation of trivalent PROTACs with controlled orientation [1] - Mechanism of action (as a PROTAC warhead): Binds to the acetyllysine-binding pocket of BET bromodomains (primarily BRD4), mediating PROTAC-induced ubiquitination and degradation of BET proteins [1] - Application: Used exclusively as an intermediate/functional component in trivalent PROTAC development for potential anticancer therapy [1] |
| 分子式 |
C19H17CLN4O2S
|
|
|---|---|---|
| 分子量 |
400.88
|
|
| 精确质量 |
400.076
|
|
| 元素分析 |
C, 56.93; H, 4.27; Cl, 8.84; N, 13.98; O, 7.98; S, 8.00
|
|
| CAS号 |
202592-23-2
|
|
| 相关CAS号 |
(+)-JQ-1;1268524-70-4;(R)-(-)-JQ1 Enantiomer; 1268524-71-5; 202592-23-2 (free); 1426257-60-4 (HCl); 2069219-37-8 (TFA); 2230314-61-9 (xTFA);
|
|
| PubChem CID |
66828107
|
|
| 外观&性状 |
Typically exists as Off-white to yellow solids
|
|
| 密度 |
1.5±0.1 g/cm3
|
|
| 沸点 |
661.6±65.0 °C at 760 mmHg
|
|
| 闪点 |
353.9±34.3 °C
|
|
| 蒸汽压 |
0.0±2.1 mmHg at 25°C
|
|
| 折射率 |
1.737
|
|
| LogP |
2.79
|
|
| tPSA |
109Ų
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
27
|
|
| 分子复杂度/Complexity |
613
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
ClC1C([H])=C([H])C(=C([H])C=1[H])C1C2C(C([H])([H])[H])=C(C([H])([H])[H])SC=2N2C(C([H])([H])[H])=NN=C2C([H])(C([H])([H])C(=O)O[H])N=1
|
|
| InChi Key |
LJOSBOOJFIRCSO-AWEZNQCLSA-N
|
|
| InChi Code |
InChI=1S/C19H17ClN4O2S/c1-9-10(2)27-19-16(9)17(12-4-6-13(20)7-5-12)21-14(8-15(25)26)18-23-22-11(3)24(18)19/h4-7,14H,8H2,1-3H3,(H,25,26)/t14-/m0/s1
|
|
| 化学名 |
2-[(9S)-7-(4-chlorophenyl)-4,5,13-trimethyl-3-thia-1,8,11,12-tetrazatricyclo[8.3.0.02,6]trideca-2(6),4,7,10,12-pentaen-9-yl]acetic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: >120 mg/mL
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.24 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.24 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1.39 mg/mL (3.47 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 1.39 mg/mL (3.47 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100μL 13.9mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 5 中的溶解度: ≥ 1.39 mg/mL (3.47 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 13.9 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4945 mL | 12.4726 mL | 24.9451 mL | |
| 5 mM | 0.4989 mL | 2.4945 mL | 4.9890 mL | |
| 10 mM | 0.2495 mL | 1.2473 mL | 2.4945 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
 Leukemia and lymphoma cell lines are broadly sensitive to BET-bromodomain inhibition.Proc Natl Acad Sci U S A.2011 Oct 4;108(40):16669-74. |
|---|
 Gene expression profiling of LP-1 and Raji cells treated with active or inactive BET inhibitors.Proc Natl Acad Sci U S A.2011 Oct 4;108(40):16669-74. |
 Small molecule BET-bromodomain inhibition suppressesMYCtranscription.Proc Natl Acad Sci U S A.2011 Oct 4;108(40):16669-74. |
 MYC reconstitution significantly protects cells from BET-mediated effects.Proc Natl Acad Sci U S A.2011 Oct 4;108(40):16669-74. |
|---|
 BET-bromodomain inhibition decreases tumor load in vivo.Proc Natl Acad Sci U S A.2011 Oct 4;108(40):16669-74. |
 Integrated genomic rationale for BET bromodomains as therapeutic targets in MM.Cell.2011 Sep 16;146(6):904-17. |
 Inhibition of Myc-dependent transcription by theJQ1BET bromodomain inhibitor.Cell.2011 Sep 16;146(6):904-17. |
|---|
 BET inhibition suppressesMYCtranscription in MM.Cell.2011 Sep 16;146(6):904-17. |
 Regulation ofMYCtranscription by BET bromodomains.Cell.2011 Sep 16;146(6):904-17. |
 Anti-myeloma activity ofJQ1in vitro.Cell.2011 Sep 16;146(6):904-17. |
|---|
 JQ1induces cell cycle arrest and cellular senescence in MM cells.Cell.2011 Sep 16;146(6):904-17. |
 Translational implications of BET bromodomain inhibition in MM.Cell.2011 Sep 16;146(6):904-17. |