| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 5g |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorbed from the small intestine by a sodium-dependent active-transport process Although the free amino acids dissolved in the body fluids are only a very small proportion of the body's total mass of amino acids, they are very important for the nutritional and metabolic control of the body's proteins. ... Although the plasma compartment is most easily sampled, the concentration of most amino acids is higher in tissue intracellular pools. Typically, large neutral amino acids, such as leucine and phenylalanine, are essentially in equilibrium with the plasma. Others, notably glutamine, glutamic acid, and glycine, are 10- to 50-fold more concentrated in the intracellular pool. Dietary variations or pathological conditions can result in substantial changes in the concentrations of the individual free amino acids in both the plasma and tissue pools. /Amino acids/ After ingestion, proteins are denatured by the acid in the stomach, where they are also cleaved into smaller peptides by the enzyme pepsin, which is activated by the increase in stomach acidity that occurs on feeding. The proteins and peptides then pass into the small intestine, where the peptide bonds are hydrolyzed by a variety of enzymes. These bond-specific enzymes originate in the pancreas and include trypsin, chymotrypsins, elastase, and carboxypeptidases. The resultant mixture of free amino acids and small peptides is then transported into the mucosal cells by a number of carrier systems for specific amino acids and for di- and tri-peptides, each specific for a limited range of peptide substrates. After intracellular hydrolysis of the absorbed peptides, the free amino acids are then secreted into the portal blood by other specific carrier systems in the mucosal cell or are further metabolized within the cell itself. Absorbed amino acids pass into the liver, where a portion of the amino acids are taken up and used; the remainder pass through into the systemic circulation and are utilized by the peripheral tissues. /Amino acids/ The intraerythrocytic malaria parasite derives much of its requirement for amino acids from the digestion of the hemoglobin of its host cell. However, one amino acid, isoleucine, is absent from adult human hemoglobin and must therefore be obtained from the extracellular medium. ... The mechanisms involved in the uptake of isoleucine by the intraerythrocytic parasite /are characterized/. Under physiologic conditions the rate of transport of isoleucine into human erythrocytes infected with mature trophozoite-stage Plasmodium falciparum parasites is increased to approximately 5-fold that in uninfected cells, with the increased flux being via the new permeability pathways (NPPs) induced by the parasite in the host cell membrane. Transport via the NPPs ensures that protein synthesis is not rate limited by the flux of isoleucine across the erythrocyte membrane. On entering the infected erythrocyte, isoleucine is taken up into the parasite via a saturable, ATP-, Na+-, and H+-independent system which has the capacity to mediate the influx of isoleucine in exchange for leucine (liberated from hemoglobin). The accumulation of radiolabeled isoleucine within the parasite is mediated by a second (high-affinity, ATP-dependent) mechanism, perhaps involving metabolism and/or the concentration of isoleucine within an intracellular organelle. Metabolism / Metabolites Hepatic The branched-chain amino acids (BCAA) -- leucine, isoleucine, and valine -- differ from most other indispensable amino acids in that the enzymes initially responsible for their catabolism are found primarily in extrahepatic tissues. Each undergoes reversible transamination, catalyzed by a branched-chain aminotransferase (BCAT), and yields alpha-ketoisocaproate (KIC, from leucine), alpha-keto-beta-methylvalerate (KMV, from isoleucine), and alpha-ketoisovalerate (KIV, from valine). Each of these ketoacids then undergoes an irreversible, oxidative decarboxylation, catalyzed by a branchedchain ketoacid dehydrogenase (BCKAD). The latter is a multienzyme system located in mitochondrial membranes. The products of these oxidation reactions undergo further transformations to yield acetyl CoA, propionyl CoA, acetoacetate, and succinyl CoA; the BCAA are thus keto- and glucogenic. Once the amino acid deamination products enter the tricarboxylic acid (TCA) cycle (also known as the citric acid cycle or Krebs cycle) or the glycolytic pathway, their carbon skeletons are also available for use in biosynthetic pathways, particularly for glucose and fat. Whether glucose or fat is formed from the carbon skeleton of an amino acid depends on its point of entry into these two pathways. If they enter as acetyl-CoA, then only fat or ketone bodies can be formed. The carbon skeletons of other amino acids can, however, enter the pathways in such a way that their carbons can be used for gluconeogenesis. This is the basis for the classical nutritional description of amino acids as either ketogenic or glucogenic (ie, able to give rise to either ketones [or fat] or glucose). Some amino acids produce both products upon degradation and so are considered both ketogenic and glucogenic. /Amino acids/ Mutations in the HSD17B10 gene were identified in two previously described mentally retarded males. A point mutation c.776G>C was found from a survivor (SV), whereas a potent mutation, c.419C>T, was identified in another deceased case (SF) with undetectable hydroxysteroid (17beta) dehydrogenase 10 (HSD10) activity. Protein levels of mutant HSD10(R130C) in patient SF and HSD10(E249Q) in patient SV were about half that of HSD10 in normal controls. The E249Q mutation appears to affect HSD10 subunit interactions, resulting in an allosteric regulatory enzyme. For catalyzing the oxidation of allopregnanolone by NAD+ the Hill coefficient of the mutant enzyme is approximately 1.3. HSD10(E249Q) was unable to catalyze the dehydrogenation of 2-methyl-3-hydroxybutyryl-CoA and the oxidation of allopregnanolone, a positive modulator of the gamma-aminobutyric acid type A receptor, at low substrate concentrations. Neurosteroid homeostasis is critical for normal cognitive development, and there is increasing evidence that a blockade of isoleucine catabolism alone does not commonly cause developmental disabilities. The results support the theory that an imbalance in neurosteroid metabolism could be a major cause of the neurological handicap associated with hydroxysteroid (17beta) dehydrogenase 10 deficiency. The HSD17B10 gene maps on chromosome Xp11.2, a region highly associated with X-linked mental retardation. This gene encodes HSD10, a mitochondrial multifunctional enzyme that plays a significant part in the metabolism of neuroactive steroids and the degradation of isoleucine. The HSD17B10 gene is composed of six exons and five introns. Its exon 5 is an alternative exon such that there are several HSD17B10 mRNA isoforms in brain. A silent mutation (c.605C-->A) and three missense mutations (c.395C-->G; c.419C-->T; c.771A-->G), respectively, cause the X-linked mental retardation, choreoathetosis, and abnormal behavior (MRXS10) and the hydroxyacyl-CoA dehydrogenase II deficiency... Human type 10 17beta-hydroxysteroid dehydrogenase (HSD) is a homotetrameric protein located in mitochondria. This enzyme was alternatively named short chain L-3-hydroxyacyl-CoA dehydrogenase (SCHSD). This NAD(H)-dependent dehydrogenase is essential for the metabolism of branched-chain fatty acids and isoleucine ... Hepatic |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
(Applies to Valine, Leucine and Isoleucine) This group of essential amino acids are identified as the branched-chain amino acids, BCAAs. Because this arrangement of carbon atoms cannot be made by humans, these amino acids are an essential element in the diet. The catabolism of all three compounds initiates in muscle and yields NADH and FADH2 which can be utilized for ATP generation. The catabolism of all three of these amino acids uses the same enzymes in the first two steps. The first step in each case is a transamination using a single BCAA aminotransferase, with a-ketoglutarate as amine acceptor. As a result, three different a-keto acids are produced and are oxidized using a common branched-chain a-keto acid dehydrogenase, yielding the three different CoA derivatives. Subsequently the metabolic pathways diverge, producing many intermediates. The principal product from valine is propionylCoA, the glucogenic precursor of succinyl-CoA. Isoleucine catabolism terminates with production of acetylCoA and propionylCoA; thus isoleucine is both glucogenic and ketogenic. Leucine gives rise to acetylCoA and acetoacetylCoA, and is thus classified as strictly ketogenic. There are a number of genetic diseases associated with faulty catabolism of the BCAAs. The most common defect is in the branched-chain a-keto acid dehydrogenase. Since there is only one dehydrogenase enzyme for all three amino acids, all three a-keto acids accumulate and are excreted in the urine. The disease is known as Maple syrup urine disease because of the characteristic odor of the urine in afflicted individuals. Mental retardation in these cases is extensive. Unfortunately, since these are essential amino acids, they cannot be heavily restricted in the diet; ultimately, the life of afflicted individuals is short and development is abnormal The main neurological problems are due to poor formation of myelin in the CNS. Interactions It has been well established that the branched chain amino acids (BCAA) compete with other large neutral amino acids (LNAA, particularly tryptophan and tyrosine) for membrane transport. Although the BCAA do not act as direct precursors for neurotransmitters, they can affect transport of certain LNAA across the blood-brain barrier, and thereby influence central nervous system concentrations of certain neurotransmitters. ... High dietary levels of leucine suppressed the growth of rats fed a low protein diet, and the growth suppression could be prevented by supplementation with isoleucine and valine. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Branched chain amino acid (BCAA)-enriched protein or amino acid mixtures and, in some cases, BCAA alone, have been used in the treatment of a variety of metabolic disorders. These amino acids have received considerable attention in efforts to reduce brain uptake of aromatic amino acids and to raise low circulating levels of BCAA in patients with chronic liver disease and encephalopathy. They have also been used in parenteral nutrition of patients with sepsis and other abnormalities. /Experimental Therapy/ Upper gastrointestinal (GI) bleeding in cirrhotic patients has a high incidence of mortality and morbidity. Postbleeding catabolism has been hypothesized to be partly due to the low biological value of hemoglobin, which lacks the essential amino acid isoleucine. The aims were to study the metabolic consequences of a "simulated" upper GI bleed in patients with cirrhosis of the liver and the effects of intravenous infusion of isoleucine. Portal drained viscera, liver, muscle, and kidney protein kinetics were quantified using a multicatheterization technique during routine portography. Sixteen overnight-fasted, metabolically stable patients who received an intragastric infusion of an amino acid solution mimicking hemoglobin every 4 hours were randomized to saline or isoleucine infusion and received a mixture of stable isotopes (L-[ring-2H5]phenylalanine, L-[ring-2H4]tyrosine, and L-[ring-2H2]tyrosine) to determine organ protein kinetics. This simulated bleed resulted in hypoisoleucinemia that was attenuated by isoleucine infusion. Isoleucine infusion during the bleed resulted in a positive net balance of phenylalanine across liver and muscle, whereas renal and portal drained viscera protein kinetics were unaffected. In the control group, no significant effect was shown... Pharmacodynamics They provide ingredients for the manufacturing of other essential biochemical components in the body, some of which are utilized for the production of energy, stimulants to the upper brain and helping you to be more alert. |
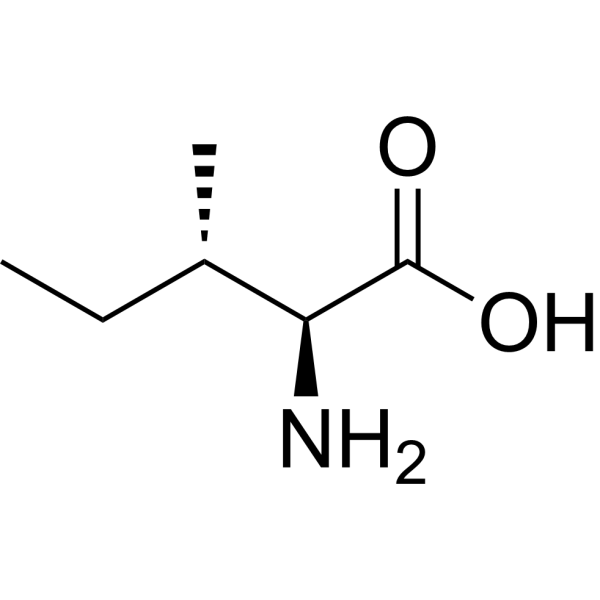
| 分子式 |
C6H13NO2
|
|---|---|
| 分子量 |
131.1729
|
| 精确质量 |
131.094
|
| CAS号 |
73-32-5
|
| 相关CAS号 |
34464-35-2
|
| PubChem CID |
6306
|
| 外观&性状 |
Waxy, shiny, rhombic leaflets from alcohol
Crystals |
| 密度 |
1.0±0.1 g/cm3
|
| 沸点 |
225.8±23.0 °C at 760 mmHg
|
| 熔点 |
168-170ºC
|
| 闪点 |
90.3±22.6 °C
|
| 蒸汽压 |
0.0±0.9 mmHg at 25°C
|
| 折射率 |
1.463
|
| LogP |
0.73
|
| tPSA |
63.32
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
9
|
| 分子复杂度/Complexity |
103
|
| 定义原子立体中心数目 |
2
|
| SMILES |
O([H])C([C@]([H])([C@@]([H])(C([H])([H])[H])C([H])([H])C([H])([H])[H])N([H])[H])=O
|
| InChi Key |
AGPKZVBTJJNPAG-WHFBIAKZSA-N
|
| InChi Code |
InChI=1S/C6H13NO2/c1-3-4(2)5(7)6(8)9/h4-5H,3,7H2,1-2H3,(H,8,9)/t4-,5-/m0/s1
|
| 化学名 |
(2S,3S)-2-amino-3-methylpentanoic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~25 mg/mL (~190.59 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 10 mg/mL (76.24 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 7.6237 mL | 38.1185 mL | 76.2369 mL | |
| 5 mM | 1.5247 mL | 7.6237 mL | 15.2474 mL | |
| 10 mM | 0.7624 mL | 3.8118 mL | 7.6237 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。