| 规格 | 价格 | |
|---|---|---|
| 100mg | ||
| 500mg |
| 体外研究 (In Vitro) |
L-酪氨酸不会改变海马 (1.0-4.0 mM) 或后皮质 (0.1-4.0 mM) 中的苹果酸脱氢酶,并且抑制后两个区域(2.0 和 4.0 mM)的柠檬酸合酶活性。纹状体 (4.0 mM)、肝脏 (0.1-4.0 mM) 和琥珀酸脱氢酶水平升高。复合物 I 活性分析显示海马区受到抑制 (4.0 mM)。复合物 II 不仅对海马体具有抑制作用,而且对肝脏(1.0、2.0 和 4.0 mM)和后皮质(0.1-4.0 mM)也具有抑制作用。 L-酪氨酸给药导致后脑中复合物 IV 的活性降低 (1.0-4.0 mM),而复合物 II-III 的活性没有变化[1]。
|
|---|---|
| 体内研究 (In Vivo) |
L-酪氨酸可显着降低肝脏和后皮质中柠檬酸合酶的活性,但增强纹状体中柠檬酸合酶的活性。研究结果还表明,在大鼠肝脏和后皮质中,L-酪氨酸的急性治疗降低了线粒体呼吸链复合物 II、III 和 IV 以及苹果酸脱氢酶的活性。纹状体显示复合物 I 和琥珀酸脱氢酶活性增加,而后皮质则显示抑制。而且,急性L-酪氨酸治疗不会改变海马能量代谢[1]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
L-tyrosine is absorbed from the small intestine by a sodium-dependent active transport process. Semi-chronic exposure of ICR male Mice to Aflatoxin B1 in non-toxic doses results in elevated lung tryptophan levels without change in serotonin or 5-hydroxyindole-3-acetic acid levels. This change is organ specific in that tryptophan levels are not altered in spleen, duodenum, heart or central nervous system. Acute (48 hr) flunixin treatment decreases lung tryptophan levels and reverses the Aflatoxin B1 mediated increase in lung tryptophan levels. On the other hand, flunixin treatment decreases central nervous system tryptophan levels in control mice but not in Aflatoxin B1 treated mice. Aflatoxin B1 treated mice have an increase in splenic serotonin content. Acute (48 hr) treatment of mice with E. coli lipopolysaccharide also increases splenic serotonin, and Aflatoxin B1 treatment followed by lipopolysaccharide have a slightly additive effect on spleen serotonin content. Treatment of mice with lipopolysaccharide increases heart serotonin, an effect which is not altered in Aflatoxin B1 pretreated mice. Both lipopolysaccharide and Aflatoxin B1 per se increases lung tyrosine levels although the combination of treatments is not significantly different from the control value. Flunixin treatment increases lung tyrosine levels, an effect which is not altered by Aflatoxin B1 pretreatment. Acute treatment with either lipopolysaccharide or flunixin decreases the central nervous system tryptophan/tyrosine ratio; pretreatment with Aflatoxin B1 prevents those changes in the central nervous system tryptophan/tyrosine ratio. Central nervous system catecholamines are reduced in Aflatoxin B1 pretreated mice. However, central nervous system catecholamine changes in Aflatoxin B1 treated mice are normalized by vitamin E supplementation during the treatment period. Male Wistar rats were divided in free choice conditions into heavy-drinkers consuming greater than 3.5 g/kg of ethanol daily, and light-drinkers consuming less than 2.0 g/kg/day. Subsequent 30 day intragastric administration of 25% ethanol (8-11 g/kg/day) caused an increase in permeability of the blood brain barrier to 14(C)-tyrosine, 14(C)-tryptophan and 14(C)-DOPA at all the stages of alcoholization. All the changes were more pronounced in light-drinkers than in heavy- drinker rats. Disulfiram, and to a lesser extent phenazepam and diazepam, when repeatedly injected (for 16-30 days) together with ethanol aggravated its effects. Effects of mercury chloride (100 uM) para-chloromercuribenzene sulfonate (1 uM), and oxophenylarsine (250 uM) were determined on (a) the rate of sodium pump activity in intact winter flounder intestine; (b) activity of sodium potassium ATPase in tissue homogenates; and (c) sodium-dependent and sodium independent uptake of tyrosine in brush border membrane vesicles. All three agents decreased cell potassium, although effects on cell potassium lagged behind those for inhibition of the ATPase. At the concentrations used in the Ussing chamber (or at one-tenth concentration), all agents completely inhibited sodium potassium ATPase activity in enzyme assays performed with tissue homogenates. In contrast, only mercury chloride decreased sodium dependent uptake of tyrosine by brush border membrane vesicles. These results suggest that mercurial and arsenical effects on tyrosine absorption are due to inhibition of the sodium potassium ATPase thus decreasing the driving force for the cellular uptake by the sodium tyrosine cotransport system. Direct effects on sodium tyrosine cotransport may play a role in the inhibition observed with mercury chloride, but not for para-chloromercuribenzene sulfonate or oxophenylarsine. Female Sprague-Dawley rats were treated acutely (12-hr) with aflatoxin B1 (100 ug/kg ip) or vehicle (10% acetone in 0.9% sodium chloride) and regional brain levels of tryptophan, serotonin and tyrosine were assayed. Brainstem but not cerebellar or cortical tyrosine levels were decreased in aflatoxin B1-treated rats. Brain tryptophan was increased in all 3 brain regions by acute aflatoxin B1 treatment, while serotonin levels were unaltered in the cerebellum and cortex and decreased in the brainstem. These experiments indicate that acute aflatoxin B1 treatment differentially alters brain amino acids and serotonin and that changes in brain tryptophan, the serotonin precursor, do not parallel changes in brain serotonin. For more Absorption, Distribution and Excretion (Complete) data for L-TYROSINE (10 total), please visit the HSDB record page. Metabolism / Metabolites In the liver, L-tyrosine is involved in a number of biochemical reactions, including protein synthesis and oxidative catabolic reactions. L-tyrosine that is not metabolized in the liver is distributed via the systemic circulation to the various tissues of the body. /METABOLIC PATHWAY FOR L-TYROSINE:/ /TYROSINE GIVES/ P-HYDROXYPHENYLPYRUVIC ACID GIVES CO2 + HOMOGENTISIC ACID GIVES MALEYLACETOACETIC ACID GIVES FUMARYLACETOACETIC ACID GIVES FUMARATE + ACETOACETATE; TYROSINE GIVES 3,4-DIHYDROXYPHENYLALANINE GIVES CO2 + 3,4-DIHYDROXYPHENYLETHYLAMINE GIVES NORADRENALIN GIVES ADRENALIN. L-TYROSINE GIVES N-ACETYL-L-TYROSINE IN MAN; GIVES 3-CARBOXY-L-TYROSINE IN RESEDA; GIVES P-COUMARIC ACID IN SUGAR CANE, L-TYROSINE GIVES PARA-CRESOL IN PROTEUS; GIVES 3,4-DIHYDROXY-L-PHENYLALANINE IN HAMSTER; GIVES 3,4-DIHYDROXYSTILBENE-2-CARBOXYLIC ACID IN HYDRANGEA, L-TYROSINE GIVES 2,7-DIMETHYLNAPHTHOQUINONE IN CHIMAPHILA; GIVES L-DITYROSINE IN BEEF; GIVES PARA-HYDROXYMANDELONITRILE IN SORGHUM, L-TYROSINE GIVES PARA-HYDROXYPHENYLACETALDOXIME IN AUBRETIA; GIVES PARA-HYDROXYPHENYLPYRUVIC ACID IN RAT; GIVES 3-IODO-L-TYROSINE IN BEEF; L-TYROSINE GIVES LACHNANTHOSIDE IN LACHNANTHES; LOPHOCERINE IN LOPHOCERUS; MESEMBRINE IN SCELETIUM; NARWEDINE IN DAFFODIL, L-TYROSINE GIVES NOVOBIOCIN IN STREPTOMYCES; PHENOL IN RAT; BETA-TOCOPHEROL IN ANABAENA; TYLOPHORINE IN TYLOPHORA, L-TYROSINE GIVES TYRAMINE IN RAT; GIVES BETA-TYROSINE IN BACILLUS; GIVES L-TYROSINE HYDROXAMATE IN BEEF. L-TYROSINE GIVES L-TYROSINE-4-PHOSPHATE IN FLY; GIVES XANTHOCILLIN IN PENICILLIUM. /FROM TABLE/ Metabolism of tyrosine was impaired after chronic alcoholization of rats with 10% ethanol within 10 months. Within the first 3-4 months activation of tyrosine aminotransferase and a decrease in phenylalanine hydroxylase activity were found in liver tissue. Activity of tyrosine aminotransferase was not increased during the long term alcohol intoxication. At the same time, activity of tyrosine aminotransferase was decreased within 5-6 months simultaneously with activation of phenylalanine hydroxylase. An increase in the alcohol dehydrogenase activity was also observed in rat liver tissue during the initial period of intoxication. The enzymatic activity was decreased beginning from the 3-4 months of the alcoholization and maintained at the low level. Hyperthermia augmented these alterations observed in chronic alcoholization of rats. Spontaneous behavior subsequent to acute oral administration of high doses of aspartame, phenylalanine, or tyrosine was analyzed using a computer pattern recognition system. Spraque Dawley male rats (250-300 g) were dosed orally with aspartame (500 or 100 mg/kg), phenylalanine (281 or 562 mg/kg), or tyrosine (309 or 618 mg/kg), and their behavior was analyzed 1 hr after dosing. The computer pattern recognition system recorded and classifed 13 different behavioral acts performed by the animals during the first 15-min exploration of a novel environment. These doses of aspartame, phenylalanine, and tyrosine did not induce any significant changes in spontaneous behavior. Unlike low doses of amphetamine and despite high plasma concentrations of phenylalanine and tyrosine, no behavioral alteration was detected by the computer pattern recognition system. For more Metabolism/Metabolites (Complete) data for L-TYROSINE (7 total), please visit the HSDB record page. In the liver, L-tyrosine is involved in a number of biochemical reactions, including protein synthesis and oxidative catabolic reactions. L-tyrosine that is not metabolized in the liver is distributed via the systemic circulation to the various tissues of the body. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Tyrosine is produced in cells by hydroxylating the essential amino acid phenylalanine. This relationship is much like that between cysteine and methionine. Half of the phenylalanine required goes into the production of tyrosine; if the diet is rich in tyrosine itself, the requirements for phenylalanine are reduced by about 50%. The mechanism of L-tyrosine's antidepressant activity can be accounted for by the precursor role of L-tyrosine in the synthesis of the neurotransmitters norepinephrine and dopamine. Elevated brain norepinephrine and dopamine levels are thought to be associated with antidepressant effects. Toxicity Data LD50 (oral, rat) > 5110 mg/kg |
| 参考文献 | |
| 其他信息 |
Pharmacodynamics
Tyrosine is a nonessential amino acid synthesized in the body from phenylalanine. Tyrosine is critical for the production of the body's proteins, enzymes and muscle tissue. Tyrosine is a precursor to the neurotransmitters norepinephrine and dopamine. It can act as a mood elevator and an anti-depressant. It may improve memory and increase mental alertness. Tyrosine aids in the production of melanin and plays a critical role in the production of thyroxin (thyroid hormones). Tyrosine deficiencies are manifested by hypothyroidism, low blood pressure and low body temperature. Supplemental tyrosine has been used to reduce stress and combat narcolepsy and chronic fatigue. |
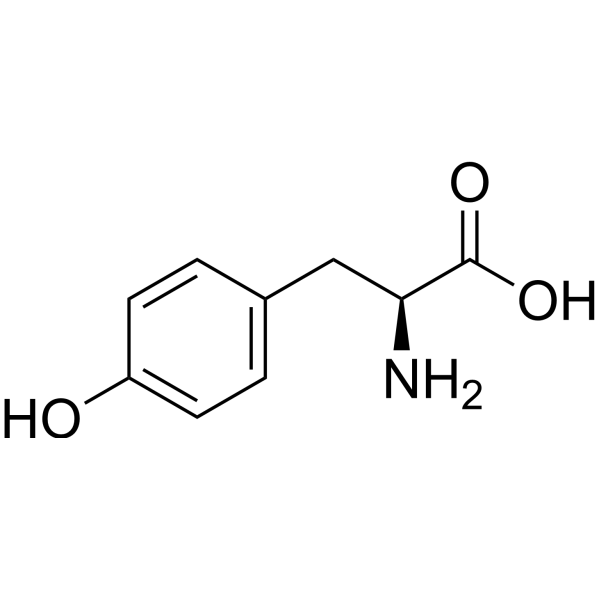
| 分子式 |
C9H11NO3
|
|---|---|
| 分子量 |
181.1885
|
| 精确质量 |
181.073
|
| CAS号 |
60-18-4
|
| 相关CAS号 |
25619-78-7
|
| PubChem CID |
6057
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
385.2±32.0 °C at 760 mmHg
|
| 熔点 |
>300 °C (dec.)(lit.)
|
| 闪点 |
186.7±25.1 °C
|
| 蒸汽压 |
0.0±0.9 mmHg at 25°C
|
| 折射率 |
1.614
|
| LogP |
0.38
|
| tPSA |
83.55
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
13
|
| 分子复杂度/Complexity |
176
|
| 定义原子立体中心数目 |
1
|
| SMILES |
C1=CC(=CC=C1C[C@@H](C(=O)O)N)O
|
| InChi Key |
OUYCCCASQSFEME-QMMMGPOBSA-N
|
| InChi Code |
InChI=1S/C9H11NO3/c10-8(9(12)13)5-6-1-3-7(11)4-2-6/h1-4,8,11H,5,10H2,(H,12,13)/t8-/m0/s1
|
| 化学名 |
(2S)-2-amino-3-(4-hydroxyphenyl)propanoic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
1M HCl : 50 mg/mL (~275.95 mM)
DMSO : ~1 mg/mL (~5.52 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 40 mg/mL (220.76 mM) in 50% PEG300 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液; 超声助溶 (<60°C).
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 40 mg/mL (220.76 mM) in 0.5% CMC-Na/saline water (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.5191 mL | 27.5953 mL | 55.1907 mL | |
| 5 mM | 1.1038 mL | 5.5191 mL | 11.0381 mL | |
| 10 mM | 0.5519 mL | 2.7595 mL | 5.5191 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。