| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 25g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
在先天感染鸭乙型肝炎病毒 (DHBV) 的小鸭产生的原代鸭肝细胞 (PDH) 培养物中,拉米夫定 (1 μM) 表现出抗病毒活性,并且是乙型肝炎病毒 (HBV) 复制的强大抑制剂[1]。拉米夫定(0-20 μM;2、4、9 d)可抑制 DHBV 复制,其 50% 抑制剂量为 0.55 μM[1]。当拉米夫定和喷昔洛韦(9-[2-羟基-1-(羟甲基)乙氧基甲基]鸟嘌呤[PCV])偶联(1 μM;2、4、9 d)时,它们表现出协同作用,特别有效地降低典型耐药病毒共价闭环 (CCC) DNA 型 DHBV[1]。
|
|---|---|
| 体内研究 (In Vivo) |
拉米夫定对大鼠肝脏有毒并产生氧化应激(20-500 mg/kg/d;口服;15 或 45 天)[2]。在易受 HIV 神经变性影响的大鼠大脑区域,拉米夫定(50 mg/kg;腹腔注射;单剂量)可有效定位并渗透中枢神经系统 (CNS)[3]。在 HIV 阳性大鼠中,拉米夫定的药代动力学参数如下: Cmax (μg/mL) 参数 Tmax (h) T1/2 (h) AUC (h·ng/mL) 血浆 25,846 0.25 0.68 22,172 脑 272 0.5 1.2 967 24小时内用于收集药代动力学数据,在用药后0.25、0.5、1.0、2.0、4.0、6.0、8.0和24.0小时采样。
|
| 动物实验 |
Animal/Disease Models: Wistar female rats[2]
Doses: 20-500 mg/kg/day Route of Administration: po (oral gavage); single or repeated dose; 15 or 45 days Experimental Results: Increased activities of the aminotransferases (ALT and AST), γ-glutamyltransferase (GGT) and total protein concentration in serum at 500 mg/kg dose. Increased activities of glutathione S-transferase (GST), GGT and superoxide dismutase (SOD) as well as concentrations of malondialdehyde (MDA) and protein at 20 mg/kg dose. Caused multifocal lymphocyte population and hepatocyte edema degeneration in hepatic sinusoids of chickens. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Lamivudine was rapidly absorbed after oral administration in HIV-infected patients. Absolute bioavailability in 12 adult patients was 86% ± 16% (mean ± SD) for the 150-mg tablet and 87% ± 13% for the oral solution. The peak serum lamivudine concentration (Cmax) was 1.5 ± 0.5 mcg/mL when an oral dose of 2 mg/kg twice a day was given to HIV-1 patients. When given with food, absorption is slower, compared to the fasted state. The majority of lamivudine is eliminated unchanged in urine by active organic cationic secretion. 5.2% ± 1.4% (mean ± SD) of the dose was excreted as the trans-sulfoxide metabolite in the urine. Lamivudine is excreted in human breast milk and into the milk of lactating rats. Apparent volume of distribution, IV administration = 1.3 ± 0.4 L/kg. Volume of distribution was independent of dose and did not correlate with body weight. Renal clearance = 199.7 ± 56.9 mL/min [300 mg oral dose, healthy subjects] Renal clearance = 280.4 ± 75.2 mL/min [single IV dose, HIV-1-infected patients] Total clearance = 398.5 ± 69.1 mL/min [HIV-1-infected patients] Lamivudine crosses the placenta and has been detected in the fetal circulation. Lamivudine has high oral bioavailability with or without food and reaches peak plasma levels within approximately 1 hour. Metabolism / Metabolites Metabolism of lamivudine is a minor route of elimination. In man, the only known metabolite of lamivudine is the trans-sulfoxide metabolite. This biotransformation is catalyzed by sulfotransferases. Biological Half-Life 5 to 7 hours (healthy or HBV-infected patients) |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Elevations in serum ALT levels occur in a proportion of patients with chronic hepatitis B treated with lamivudine. These elevations appear to be due to a transient flare in the underlying chronic hepatitis B and occur in three situations during and after therapy: upon initiation of therapy (treatment flares), upon development of antiviral resistance (breakthrough flares), and shortly after stopping therapy (withdrawal flares). Treatment flares typically occur during the first few months of therapy and are marked by asymptomatic elevations in serum aminotransferase levels and rarely with jaundice or symptoms (Case 1). These flares occur during the rapid decrease in HBV DNA levels with initiation of therapy. An exacerbation of hepatitis also typically occurs after development of lamivudine resistance, a few weeks or months after the initial appearance of the mutant HBV strain and rise in HBV DNA levels (Case 2). Finally, withdrawal flares occur between 4 and 12 weeks after stopping lamivudine and can be severe, symptomatic and even lead to clinical decompensation, acute liver failure and either death or need for emergency liver transplantation. Resistance and withdrawal flares typically occur as HBV DNA levels are high or rising. Other forms of hepatotoxicity from lamivudine are extremely rare if they occur at all. Lamivudine is a rare cause of liver test abnormalities or clinically apparent liver injury in patients with HIV infection without hepatitis B. Although several instances of lactic acidosis with hepatic steatosis and liver failure have been reported in patients receiving lamivudine, in all instances other nucleoside analogues more clearly associated with mitochondrial injury [didanosine, stavudine, zalcitrabine, zidovudine] were also being taken. No convincing instances of lactic acidosis with microvesicular fat have been reported in patients with hepatitis B who typically receive lamivudine alone or in combination with adefovir or tenofovir. Likelihood score: E (unlikely cause of clinically apparent liver injury although flares of hepatitis B can occur during or following therapy). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Lamivudine has been well studied in HIV-positive nursing mothers and appears to be well tolerated by their breastfed infants. It has not been studied in HIV-negative nursing mothers being treated for hepatitis B infection, but the low doses used would not be expected to cause any serious adverse effects in breastfed infants. The manufacturer estimates that a breastfed infant's dose would be about 6% of the infant dose for children over 2 years of age. Achieving and maintaining viral suppression with antiretroviral therapy decreases breastfeeding transmission risk to less than 1%, but not zero. Individuals with HIV who are on antiretroviral therapy with a sustained undetectable viral load and who choose to breastfeed should be supported in this decision. If a viral load is not suppressed, banked pasteurized donor milk or formula is recommended. An expert review of available data concluded that there is currently no justification for contraindicating the use of lamivudine for hepatitis B therapy during breastfeeding. Some professional organization guidelines allow breastfeeding during lamivudine therapy, although one guideline cautions against it because of a lack of long-term safety data. The lack of long-term safety data with long-term, low-level infant exposure should be discussed with the mother. No differences exist in infection rates between breastfed and formula-fed infants born to hepatitis B-infected women, as long as the infant receives hepatitis B immune globulin and hepatitis B vaccine at birth. Mothers with hepatitis B are encouraged to breastfeed their infants after their infants receive these preventative measures. ◉ Effects in Breastfed Infants A study assigned pregnant women to zidovudine alone or highly active antiretroviral therapy (HAART: zidovudine, lamivudine and nevirapine) to prevent maternal-to-child transmission of HIV infection. After delivery, all infants received one month of zidovudine prophylaxis; some infants were breastfed and others were formula fed. A higher percentage of infants in the HAART-exposed group had neutropenia than those in the unexposed group at 1 month of age (15.9% and 3.7%, respectively). Hematologic toxicity was transient and asymptomatic. From 2 to 6 months postpartum, no differences in hematologic toxicity were seen between breastfed and formula-fed infants. No statistical difference in hepatic toxicity was seen between the breastfed and formula-fed infants. Twenty-four infants who were breastfed by HIV-positive mothers developed HIV infection by 6 months of age. Six of these infants had a mutation that might have been selected for by subtherapeutic levels of lamivudine in breastmilk. An HIV-positive mother took a combination tablet containing dolutegravir 50 mg, abacavir sulfate 600 mg and lamivudine 300 mg (Triumeq) once daily. Her infant was exclusively breastfed for about 30 weeks and partially breastfed for about 20 weeks more. No obvious side effects were noted. One mother took lamivudine for 33 days, 25 before birth and eight days postpartum for chronic hepatitis B infection. Her infant was breastfed (extent not stated). At three months of age, the infant died with the death attributed to sudden infant death syndrome. The death was unlikely to be related to lamivudine. ◉ Effects on Lactation and Breastmilk Gynecomastia has been reported among men receiving highly active antiretroviral therapy. Gynecomastia is unilateral initially, but progresses to bilateral in about half of cases. No alterations in serum prolactin were noted and spontaneous resolution usually occurred within one year, even with continuation of the regimen. Some case reports and in vitro studies have suggested that protease inhibitors might cause hyperprolactinemia and galactorrhea in some male patients, although this has been disputed. The relevance of these findings to nursing mothers is not known. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Protein Binding <36% bound to plasma protein. |
| 参考文献 | |
| 其他信息 |
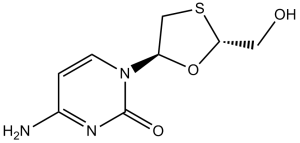
Lamivudine is a monothioacetal that consists of cytosine having a (2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl moiety attached at position 1. An inhibitor of HIV-1 reverse transcriptase, it is used as an antiviral in the treatment of AIDS and hepatitis B. It has a role as a HIV-1 reverse transcriptase inhibitor, an antiviral drug, an anti-HBV agent, an allergen, a prodrug and an EC 2.7.7.49 (RNA-directed DNA polymerase) inhibitor. It is a monothioacetal, a primary alcohol, an oxacycle and a nucleoside analogue. It is functionally related to a cytosine.
Lamivudine (brand name: Epivir) is a prescription medicine approved by the U.S. Food and Drug Administration (FDA) for the treatment of HIV infection in adults and children. Lamivudine is always used in combination with other HIV medicines. A reverse transcriptase inhibitor and zalcitabine analog in which a sulfur atom replaces the 3' carbon of the pentose ring. It is used to treat Human Immunodeficiency Virus Type 1 (HIV-1) and hepatitis B (HBV). Lamivudine is a Hepatitis B Virus Nucleoside Analog Reverse Transcriptase Inhibitor and Human Immunodeficiency Virus Nucleoside Analog Reverse Transcriptase Inhibitor. The mechanism of action of lamivudine is as a Nucleoside Reverse Transcriptase Inhibitor. Lamivudine is a nucleoside analogue and reverse transcriptase inhibitor used in the therapy of human immunodeficiency virus (HIV) and hepatitis B virus (HBV) infection. Lamivudine is a very rare cause of clinically apparent drug induced liver injury, but is associated with flares of underlying hepatitis B during therapy or with abrupt withdrawal. Lamivudine has been reported in Schisandra bicolor and Vitex tripinnata with data available. Lamivudine is a synthetic nucleoside analogue with activity against hepatitis B virus (HBV) and HIV. Intracellularly, lamivudine is phosphorylated to its active metabolites, lamiduvine triphosphate (L-TP) and lamiduvine monophosphate (L-MP). In HIV, L-TP inhibits HIV-1 reverse transcriptase (RT) via DNA chain termination after incorporation of the nucleoside analogue into viral DNA. In HBV, incorporation of L-MP into viral DNA by HBV polymerase results in DNA chain termination. L-TP is a weak inhibitor of mammalian DNA polymerases alpha and beta, and mitochondrial DNA polymerase. (NCI04) A reverse transcriptase inhibitor and ZALCITABINE analog in which a sulfur atom replaces the 3' carbon of the pentose ring. It is used to treat HIV disease. Drug Indication For the treatment of HIV infection and chronic hepatitis B (HBV). FDA Label Lamivudine Teva Pharma B. V. is indicated as part of antiretroviral combination therapy for the treatment of human-immunodeficiency-virus (HIV)-infected adults and children. Lamivudine Teva is indicated for the treatment of chronic hepatitis B in adults with: compensated liver disease with evidence of active viral replication, persistently elevated serum alanine aminotransferase (ALT) levels and histological evidence of active liver inflammation and / or fibrosis. Initiation of lamivudine treatment should only be considered when the use of an alternative antiviral agent with a higher genetic barrier is not available or appropriate (see in section 5. 1). Epivir is indicated as part of antiretroviral combination therapy for the treatment of human-immunodeficiency-virus (HIV)-infected adults and children. , Zeffix is indicated for the treatment of chronic hepatitis B in adults with: , , , compensated liver disease with evidence of active viral replication, persistently elevated serum alanine aminotransferase (ALT) levels and histological evidence of active liver inflammation and / or fibrosis. Initiation of lamivudine treatment should only be considered when the use of an alternative antiviral agent with a higher genetic barrier is not available or appropriate; , decompensated liver disease in combination with a second agent without cross-resistance to lamivudine. , , Mechanism of Action Lamivudine is a synthetic nucleoside analogue and is phosphorylated intracellularly to its active 5'-triphosphate metabolite, lamivudine triphosphate (L-TP). This nucleoside analogue is incorporated into viral DNA by HIV reverse transcriptase and HBV polymerase, resulting in DNA chain termination. Lamivudine enters cells by passive diffusion and is phosphorylated to its active metabolite, lamivudine triphosphate. Lamivudine triphosphate competes with deoxycytidine triphosphate for binding to reverse transcriptase, and incorporation into DNA results in chain termination. Lamivudine has very low affinity for human alpha and omega DNA polymerases, moderate affinity for beta DNA polymerase, and higher affinity for gamma DNA polymerase. |
| 分子式 |
C8H11N3O3S
|
|
|---|---|---|
| 分子量 |
229.26
|
|
| 精确质量 |
229.052
|
|
| CAS号 |
134678-17-4
|
|
| 相关CAS号 |
Lamivudine salicylate;173522-96-8
|
|
| PubChem CID |
60825
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.7±0.1 g/cm3
|
|
| 沸点 |
475.4±55.0 °C at 760 mmHg
|
|
| 熔点 |
177 °C
|
|
| 闪点 |
241.3±31.5 °C
|
|
| 蒸汽压 |
0.0±2.7 mmHg at 25°C
|
|
| 折射率 |
1.755
|
|
| LogP |
-0.71
|
|
| tPSA |
115.67
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
15
|
|
| 分子复杂度/Complexity |
331
|
|
| 定义原子立体中心数目 |
2
|
|
| SMILES |
C1[C@H](O[C@H](S1)CO)N2C=CC(=NC2=O)N
|
|
| InChi Key |
JTEGQNOMFQHVDC-NKWVEPMBSA-N
|
|
| InChi Code |
InChI=1S/C8H11N3O3S/c9-5-1-2-11(8(13)10-5)6-4-15-7(3-12)14-6/h1-2,6-7,12H,3-4H2,(H2,9,10,13)/t6-,7+/m0/s1
|
|
| 化学名 |
4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (10.90 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (10.90 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (10.90 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 100 mg/mL (436.19 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.3619 mL | 21.8093 mL | 43.6186 mL | |
| 5 mM | 0.8724 mL | 4.3619 mL | 8.7237 mL | |
| 10 mM | 0.4362 mL | 2.1809 mL | 4.3619 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。