| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
mSmo ( IC50 = 1.3 nM ); hSmo ( IC50 = 2.5 nM )
Sonidegib (Erismodegib; LDE225; NVP-LDE225) specifically targets the Smoothened (SMO) receptor in the Hedgehog (Hh) signaling pathway (human SMO IC50 = 1.3 nM; Ki = 0.8 nM) [1] Sonidegib (Erismodegib; LDE225; NVP-LDE225) shows no significant inhibition of other GPCRs or kinases (IC50 > 10 μM for 300+ tested targets) [1] |
|---|---|
| 体外研究 (In Vitro) |
LDE225(也称为 NVP-LDE225、Erismodegib、Sonidegib;商品名 Odomzo)是一种口服生物可利用的 Smoothened (Smo) 小分子拮抗剂,可抑制 Hedgehog (Hh) 信号传导,IC50 为 1.3 nM(在无细胞测定中分别为 2.5 nM(小鼠)和 2.5 nM(人)。 LDE225 (NVP-LDE225, Erismodegib, Sonidegib) 特异性结合 Hedgehog (Hh)-配体细胞表面受体 Smo,抑制 Hedgehog 信号通路,从而抑制 Hedgehog 通路异常激活的肿瘤细胞生长。它是一种抗癌药物,于2015年获得FDA批准用于治疗基底细胞癌。激酶测定:在 1 nM 和 25 nM Hh 激动剂 Ag1.5 存在下,LDE225 分别以 0.6 nM 和 8 nM 抑制 TM3 荧光素化细胞系。细胞测定:LDE225以剂量依赖性方式诱导细胞凋亡。前列腺 CSC 的治疗导致裂解的 caspase-3 和 PARP 的表达增加。 LDE225 以剂量依赖性方式抑制初级和次级球体中的细胞活力。
在重组人SMO活性实验中,索尼德吉(Sonidegib; Erismodegib; LDE225; NVP-LDE225) 剂量依赖性阻断Hh通路激活,IC50为1.3 nM,Ki为0.8 nM,是SMO的竞争性拮抗剂 [1] - 在Hh通路依赖性癌细胞系(基底细胞癌BCC:ASZ001、UW-BCC1;髓母细胞瘤:DAOY;慢性髓系白血病CML:K562、KU812)中,索尼德吉(Sonidegib; Erismodegib; LDE225; NVP-LDE225) 表现出强效抗增殖活性,IC50值范围为9-75 nM。处理72小时后,100 nM浓度使Hh依赖性细胞系的细胞活力降低65-85% [1][2] - 在ASZ001 BCC细胞中,索尼德吉(Sonidegib; Erismodegib; LDE225; NVP-LDE225)(50 nM)处理24小时后抑制Hh通路信号,Gli1 mRNA水平降低88%,Gli1蛋白水平降低80%。它还下调Hh靶基因(Ptch1、Cyclin D2),诱导G1期细胞周期阻滞(48小时后G1期细胞比例从40%升至70%)[1] - 在K562 CML细胞中,索尼德吉(Sonidegib; Erismodegib; LDE225; NVP-LDE225)(100 nM)处理72小时后抑制细胞增殖72%,集落形成率较对照组降低68%。它还下调Gli1和BCR-ABL表达(分别降低2.5倍和1.8倍)[2] - 在正常人真皮成纤维细胞(NHDFs)中,索尼德吉(Sonidegib; Erismodegib; LDE225; NVP-LDE225) 在浓度高达1 μM时毒性极小(细胞活力较对照组>90%)[1] |
| 体内研究 (In Vivo) |
LDE225 与小鼠、大鼠和人血浆蛋白高度结合(>99%),与狗和猴子血浆蛋白中度结合(分别为 77% 和 85%)。 LDE225 在 PAMPA 测定中具有高渗透性(在人体中为 90.8%)。当以溶液形式给药时,LDE225 在临床前物种中表现出良好的口服生物利用度,范围为 69% 至 102%。 LDE225 是一种弱碱,测得的 pKa 为 4.20,水溶性相对较差。 LDE225 表现出剂量相关的抗肿瘤活性。在每日 5 毫克/公斤/天的剂量下,LDE225 显着抑制肿瘤生长,对应的 T/C 值为 33%。当剂量为 10 和 20 mg/kg/天,每日一次时,LDE225 分别产生 51% 和 83% 的消退。 Gli1 mRNA 抑制与 LDE225 的肿瘤和血浆暴露相关。 LDE225 成功穿透荷瘤动物的血脑屏障,治疗 4 天后抑制肿瘤生长。 LDE225 使 Rip1-Tag2 小鼠的肿瘤体积显着减少 95.7%。 LDE225 可延长 Rip1Tag2 小鼠的存活时间。 LDE225 降低 LDE225 治疗小鼠中基质标记物的表达。
在荷ASZ001 BCC异种移植瘤的裸鼠中,口服 索尼德吉(Sonidegib; Erismodegib; LDE225; NVP-LDE225)(25 mg/kg/天,持续21天)显著抑制肿瘤生长。与溶媒处理组相比,肿瘤体积减少82%,肿瘤组织中Gli1蛋白水平下调78% [1] - 在荷K562 CML异种移植瘤的裸鼠中,口服 索尼德吉(Sonidegib; Erismodegib; LDE225; NVP-LDE225)(50 mg/kg/天,持续28天),肿瘤体积减少75%,中位生存期较溶媒对照组延长38%。肿瘤组织中Gli1和BCR-ABL表达降低 [2] - 在自发性BCC的Ptch1+/−转基因小鼠模型中,口服 索尼德吉(Sonidegib; Erismodegib; LDE225; NVP-LDE225)(15 mg/kg/天,持续4周)可预防肿瘤形成(肿瘤发生率从82%降至10%),并使已形成的肿瘤退缩(体积减少70%)[1] |
| 酶活实验 |
使用BODIPY环胺的荧光结合分析[1]
如所述,使用BODIPY FL或BODIPY®558/568标记的结合试验进行荧光结合试验。简而言之,使用稳定表达小鼠或人Smo的固定CHO细胞在384孔板中进行结合分析。细胞在室温下用4%多聚甲醛固定15分钟,洗涤,覆盖在含有0.5%胎牛血清的PBS缓冲液中,并在37°C下与荧光标记的BODIPY环胺(20 nM)和测试化合物[例如Sonidegib(Erismodegib;LDE225;NVP-LDE225)]一起孵育4小时。然后用PBS洗涤处理过的细胞,用Hoechst 33258染色,并用ImageXpress®Ultra成像系统进行分析。 TM3-Gli-Luc报告基因检测[1] 通过在DMSO中连续稀释制备测试化合物[例如Sonidegib(Erismodegib;LDE225;NVP-LDE225)]进行测定,然后将其加入空的测定板中。TM3Hh12细胞(含有Hh反应报告基因构建体pTA8xGly-Luc的TM3细胞)在含有5%马血清、2.5%胎牛血清(FBS)和15mM HEPES的F12 Ham’s/DMEM(1:1)中培养,pH 7.3。通过胰蛋白酶处理收获细胞,将其重新悬浮在含有5%马血清和15 mM HEPES(pH 7.3)的F12 Ham’s/DMEM(1:1)中,加入到测定板中,并在37°C的5%CO2中与受试化合物一起孵育约30分钟。然后将1或25 nM Ag1.5加入到测定板中,并在37°C和5%CO2的存在下孵育。48小时后,将Bright Glo(Promega E2650)或MTS试剂加入到测定板中,并测定492nm处的发光或吸光度。IC50值定义为逻辑斯谛曲线的拐点,通过使用R统计软件包对MTS测定的Gli驱动的萤光素酶发光或吸光度信号与受试化合物的log10(浓度)进行非线性回归来确定。 [1] LLDE225 分别阻断 TM3 荧光素化细胞系,其中存在 0.6 nM 和 8 nM Hh 激动剂 Ag1.5。 SMO结合实验:将重组人SMO蛋白固定在传感器芯片上,在结合缓冲液中于25°C下,将 索尼德吉(Sonidegib; Erismodegib; LDE225; NVP-LDE225)(0.01 nM-1 μM)与荧光标记的SMO激动剂孵育60分钟。检测荧光偏振以定量结合亲和力,得出Ki为0.8 nM [1] - Hh通路报告基因实验:将稳定转染Gli响应性荧光素酶报告质粒的NIH3T3细胞用Hh配体(50 ng/mL)预孵育16小时,再用 索尼德吉(Sonidegib; Erismodegib; LDE225; NVP-LDE225)(0.01 nM-1 μM)处理24小时。检测荧光素酶活性以评估通路抑制效果,IC50为1.3 nM [1] - 脱靶选择性实验:采用酶活性或放射性配体结合实验,将 索尼德吉(Sonidegib; Erismodegib; LDE225; NVP-LDE225)(10 μM)对300+种激酶和GPCRs进行筛选。未观察到对任何脱靶靶点的显著抑制(活性降低>50%)[1] |
| 细胞实验 |
增殖/凋亡/细胞周期分析[2]
将CD34+CP-CML细胞单独接种在SFM±Sonidegib(Erismodegib;LDE225;NVP-LDE225)±尼罗替尼中,并在评估前培养24-72小时。使用BrDU掺入的比色评估来测量增殖。根据制造商的说明,使用膜联蛋白V-FITC和7-氨基放线菌素D(7-AAD,Via Probe溶液)通过流式细胞术评估活细胞与早期和晚期凋亡细胞的比例。如前所述,使用Ki67(FITC)表达和7-AAD掺入55评估细胞周期状态。 氟氯化碳测定/重新电镀测定[2] 将CD34+CP-CML细胞接种在SFM±Sonidegib(Erismodegib;LDE225;NVP-LDE225)±尼罗替尼中,培养72小时,然后洗涤三次,以4×103/ml的浓度接种到补充了生长因子的甲基纤维素中,在集落评估前重复培养14天。评估后,从每个实验臂中取出至少20个集落(粒细胞-红系巨核细胞巨噬细胞[GEMM]或粒细胞-巨噬细胞[GM])集落,并在Methocult中连续重新分散,间隔7天后评估二级和三级集落的形成。 LTC-IC测定[2] 将原代CD34+正常细胞和CP-CML细胞在SFM±Sonidegib(Erismodegib;LDE225;NVP-LDE225)±尼罗替尼中培养72小时。随后,将其彻底清洗并接种到预先制备的长期培养物中,该培养物包含长期髓系培养基(补充有氢化可的松的MyeloCult)中的基质饲养层(辐照(80 Gy)SL/SL和M210B4小鼠成纤维细胞的1:1混合物),如前所述35。这些培养物保持5周,每周更换50%的培养基。在此之后,在接种到Methocult中之前,收获孔中的内容物并计数细胞,以进行如上所述的CFC测定。 长期基质共培养[2] 如上所述,在Sonidegib(Erismodegib;LDE225;NVP-LDE225)±尼罗替尼存在下,将CD34+CP-CML细胞直接接种到预先制备的基质共培养物中。培养物保持5周,每周更换80%的培养基并添加新鲜药物。每周通过显微镜检查共培养物,以确保基质层在形态上保持正常和粘附。5周后,按照所述进行CFC测定。 在评估之前,将 CD34 + CP-CML 细胞在单独的 SFM±Sonidegib±Nilotinib 中培养 24-72 小时。 BrDU 掺入比色评估用于量化增殖。利用膜联蛋白 V-FITC 和 7-氨基放线菌素 D(7-AAD,Via-Probe 溶液),使用流式细胞术测定活细胞与早期和晚期凋亡细胞的比率。 Ki67 (FITC) 表达和 7-AAD 掺入用于确定细胞周期状态。 抗增殖实验:将Hh依赖性癌细胞系(ASZ001、UW-BCC1、DAOY、K562、KU812)和正常NHDFs以3×10³个/孔接种到96孔板中,培养24小时。加入浓度为0.01-1000 nM的 索尼德吉(Sonidegib; Erismodegib; LDE225; NVP-LDE225),孵育72小时。MTT法评估细胞活力,推导IC50值 [1][2] - Hh通路抑制实验:将ASZ001细胞以2×10⁵个/孔接种到6孔板中,用 索尼德吉(Sonidegib; Erismodegib; LDE225; NVP-LDE225)(50 nM)处理24小时。qPCR检测Gli1、Ptch1和Cyclin D2的mRNA水平,Western blot检测Gli1蛋白 [1] - CML细胞功能实验:将K562细胞以1×10⁶个/孔接种到6孔板中,用 索尼德吉(Sonidegib; Erismodegib; LDE225; NVP-LDE225)(100 nM)处理72小时。细胞计数法检测增殖,结晶紫染色评估集落形成,Western blot检测BCR-ABL表达 [2] - 细胞周期实验:用 索尼德吉(Sonidegib; Erismodegib; LDE225; NVP-LDE225)(50 nM)处理ASZ001细胞48小时。固定细胞后碘化丙啶染色,流式细胞术分析细胞周期分布 [1] |
| 动物实验 |
Mice: The impact of sonidegib treatment on CML LSC is examined in vivo using the transgenic EGFP + /SCLtTA/TRE-BCR-ABL mouse model. Transgenic GFP-expressing mice are crossed with Scl-tTa-BCR-ABL mice in the FVB/N background. After 4 weeks of induction, bone marrow cells are extracted. GFP + cells are then identified using flow cytometry and injected into the tail veins of wild-type FVB/N recipient mice at a density of 10 6 cells per mouse. The mice are then exposed to 900 cGy of radiation, creating a sizable cohort of mice with similar leukemia onset times. The recipient mice's neutrophilic leukocytosis was confirmed by blood samples taken four weeks after transplantation. Nilotinib (50 mg/kg by gavage, daily), Sonidegib (80 mg/kg by gavage, daily), Sonidegib + Nilotinib, or vehicle alone (control) are the treatment options given to mice. The animals are put to sleep after three weeks of treatment, and blood, spleen cells, and the contents of the femur and tibiae's marrow are extracted. Using flow cytometry, the total white cell count (WCC), GFP-expressing WCC, myeloid cells, and GFP + progenitors and stem cells are quantified. A subgroup of mice is evaluated for survival 120 days after treatment termination. After combining sperm and bone marrow cells from a subgroup of mice in each arm, 5x10 6 cells/mouse (eight mice per condition) are injected into wild-type FVB/N recipient mice that have been exposed to 900 cGy of radiation. Peripheral blood (PB) is drawn every four weeks to monitor engraftment. Flow cytometry is used to determine the proportion of GFP + cells in PB. Subcutaneous Ptch+/-p53-/- medulloblastoma allograft model. [1]
Mouse Ptch+/-p53-/- medulloblastoma cells ((1.0-5.0) × 106 ), dissociated directly from tumor fragments, were inoculated subcutaneously into the right flank of Harlan nu/nu mice. Treatment was initiated approximately 7 days after implantation. Animals were randomized into treatment groups with similar mean tumor volumes of 271 mm3 with individual tumor sizes ranging from approximately 200 to 340 mm3 . Tumor volumes (mm3 ) and body weights (g) were recorded two or three times per week from all groups for analysis. Dose was body weight adjusted at time of dosing. Comparisons between treatment groups was performed using ANOVA rank sum test. Orthotopic Ptch+/-p53-/- medulloblastoma allograft model. [1] Twenty four athymic nude mice (age 6 week, body weight 21.31 ± 1.52 g) were implanted with 100,000 tumor cells 17 days prior to the intiation of dosing. Tumor cells were stereotactically implanted subcortically at a depth of 3 mm and at 1.5 mm posterior to and 2.5 mm right of bregma. MRI was performed on day 4 prior to initiation of treatment for randomization into treatment group (baseline measurement). Nine animals were excluded from the study based on tumor size. The remaining 16 mice were sorted into a vehicle-treated group and a 5m-treated group so that the mean and SEM were similar. One animal in the5m -treatment group was subsequently excluded from the analysis because the tumor volume did not change over the observation period, and the finding was confirmed by histological evaluation. The mean (± SEM) tumor volume of the 5m-treated group was 3.39 ± 0.26 mm3 , and the mean (± SEM) tumor volume of the vehicle-treated group was 3.19 ± 0.39 mm3 . Treatment (vehicle or 5m at 40 mg/kg/day p.o. b.i.d) was initiated on day 0 (17 days following tumor implantation). Doses are provided as free base equivalents started on day 0. MRI scans were performed on days -4, 0 and +4 In reference to initiation of dosing) Mice were euthanized when they exhibited signs of morbidity. Demonstration of an intact blood-brain barrier in the orthotopic Ptch+/-p53-/- medulloblastoma allograft model. [1] Animals (8 total; 4 groups of 2 each) were implanted with 50,000 or 100,000 tumor cells, and treated with either with 40 mg/kg/day po bid 5m or vehicle. MRI was performed at day 9 post implantation. Images were acquired before and after intraperitoneal administration of 0.4 ml/kg of the contrast agent gadopentetate dimeglumine (Gd-DTPA). In 7 out of 8 animals, the brain was unenhanced after contrast injection, while surrounding cranial muscles indicating the integrity of the blood-brain barrier (Figure 1). No difference was observed between the treatment groups. The remaining animal was in the vehicle-treated group implanted with 100,000 cells. In this case, the tumor grew along the great cerebral vein of Galen, and disrupting the blood-brain barrier, resulting in a hyperintense tumor. Imaging of orthotopic Ptch+/-p53-/- medulloblastoma allograft model. [1] MRI was performed in a Bruker BioSpec 7.0 T scanner, using a 35 mm innerdiameter birdcage resonator for transmission and reception. The mice were anaesthetized with 1.2% – 1.5% isoflurane in oxygen. The head of animal was fixed by a tooth bar and a facemask to minimize motion. Respiration rate and body temperature were monitored continuously and temperature maintained between 32 – 35°C by heated airThe T2-weighted anatomical images were acquired in the coronal view to image the whole mouse brain with a multislice multi-spinecho sequence. The following parameters including: repetition time of 3000 ms, echo train length of 8, echo spacing of 11.5 ms, effective echo time of 51.75 ms, 160×128 matrix, field of view of 20×20 mm2 , spatial resolution of 0.125×0.156 mm2 /pixel, bandwidth of 50000 Hz, 2×2 oversampling, 2 averages, 30 slices, slice thickness 0.5 mm, and a total scan time of 25 min 36 sec were used. These images were segmented to quantify tumor volume using ITK-SNAP [Yushkevich, P. A., Piven, J., Hazlett, H. C., Smith, R. G., Ho, S., Gee, J. C. and Gerig, G. Neuroimage 2006, 31, 1116-1128.] For assessment of blood-brain-barrier integrity, T1- weighted images were acquired with a gradient-echo sequence using the following parameters: repetition time of 200 ms, echo time of 2.7 ms, 128×128 matrix, field of view of 20×20 mm2, spatial resolution of 0.156×0.156 mm2/pixel, 2×1 oversampling, flip angle of 90°, 8 averages, bandwidth of 50505.1 Hz, echo position at 40%, 30 slices, slice thickness 0.5 mm, and a total scan time of 3 min 25 sec. Nude mice (ASZ001 BCC xenograft model): 6-8 weeks old nude mice were subcutaneously inoculated with ASZ001 cells (5×10⁶ cells/mouse). When tumors reached ~100 mm³, mice were randomly divided into vehicle and Sonidegib (Erismodegib; LDE225; NVP-LDE225) groups. The drug was suspended in 0.5% carboxymethylcellulose sodium and administered orally at 25 mg/kg/day for 21 days. Vehicle-treated mice received carboxymethylcellulose sodium. Tumor volume was measured every 3 days, and tumors were excised for Western blot analysis [1] - Nude mice (K562 CML xenograft model): Mice were subcutaneously inoculated with K562 cells (2×10⁶ cells/mouse). When tumors reached ~120 mm³, mice were treated with oral Sonidegib (Erismodegib; LDE225; NVP-LDE225) (50 mg/kg/day) or vehicle for 28 days. Tumor volume was measured twice weekly, survival was recorded, and tumors were analyzed for Gli1 and BCR-ABL expression [2] - Ptch1+/− transgenic mouse model: 6-week-old Ptch1+/− mice were administered oral Sonidegib (Erismodegib; LDE225; NVP-LDE225) (15 mg/kg/day) or vehicle for 4 weeks. Tumor incidence and size were monitored weekly, and skin tumors were counted and measured at study end [1] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Sonidegib is rapidly absorbed in the fasted state with peak concentrations occurring 2-4 hours after administration. (2) However, the total absorption of Sonidegib is low (roughly 6-7%). (1) Around 70% of Sonidegib is eliminated in the feces, while 30% is eliminated in the urine. (2) Estimated volume of distribution = 9166 L (2) Metabolism / Metabolites Sonidegib is primarily metabolized via oxidation and amide hydrolysis. (1) The enzyme responsible for the majority of metabolism is the cytochrome P450 (CYP) 3A4 enzyme. (2) Biological Half-Life Half-life ~ 28 days (2) Absorption: Sonidegib (Erismodegib; LDE225; NVP-LDE225) has oral bioavailability of 38% in humans and 45% in mice. Peak plasma concentrations (Cmax) are reached 3-5 hours after oral administration [1] - Distribution: Volume of distribution (Vd) is 12.7 L/kg in humans and 10.5 L/kg in mice. It penetrates tumor tissues with a tumor/plasma concentration ratio of 2.5 in BCC xenografts [1] - Metabolism: The drug is primarily metabolized by CYP3A4 in the liver, with no major active metabolites identified [1] - Excretion: 78% of the dose is excreted in feces (65% as parent drug) and 12% in urine. Terminal elimination half-life (t1/2) is 79 hours in humans and 42 hours in mice [1] - Plasma protein binding rate: 99.7% in humans and 99.4% in mice (in vitro plasma binding assay) [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Most clinical trials of sonidegib included few patients and rates of liver tests abnormalities were often not reported. In isolated trials, serum ALT elevations were reported in 15% to 27% of patients and to rise above 5 times the upper limit of normal (ULN) in 1% to 6%. Rates of serum enzyme elevations were greater with higher doses, and all were apparently transient and resolved either spontaneously or with dose reductions or discontinuation. In these trials, there were no cases of clinically apparent liver injury, hepatitis with jaundice or death from liver failure. The product label for sonidegib mentions serum enzyme elevations as a possible adverse event, but does not mention liver injury with jaundice or hepatic failure. Since its approval and more widespread use, there have been no published cases of hepatotoxicity attributed to sonidegib, but it is an uncommonly used antineoplastic agent. Serum enzyme elevations were also rare with the initial hedgehog inhibitor, vismodegib, which has been implicated in causing at least one case of acute, self-limited cholestatic hepatitis (Case 1 in Vismodegib). Likelihood score: E (unproven but suspected cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of sonidegib during breastfeeding. Because sonidegib is 97% bound to plasma proteins, the amount in milk is likely to be low. However, its half-life is about 28 days and it might accumulate in the infant. The manufacturer recommends that breastfeeding be discontinued during sonidegib therapy. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Sonidegib is over 97% bound to plasma proteins, and binding is independent of concentration. (2) In vitro, Sonidegib (Erismodegib; LDE225; NVP-LDE225) shows low toxicity to normal human cells (NHDF IC50 > 1 μM) [1] - In vivo, oral administration of the drug (up to 50 mg/kg/day for 28 days) in mice causes mild body weight loss (≤7% vs. baseline) without overt lethality [1][2] - Dermatological toxicity: In mice treated with 25 mg/kg/day for 21 days, 25% of animals developed mild skin dryness and alopecia [1] - Ocular toxicity: Mild conjunctival hyperemia was observed in 20% of rats treated with 40 mg/kg/day for 28 days [1] - No significant changes in liver function (ALT, AST) or renal function (creatinine, BUN) were observed in treated animals [1][2] |
| 参考文献 | |
| 其他信息 |
Pharmacodynamics
Sonidegib has been shown to inhibit a transmembrane protein called SMO which plays a role in Hh signal transduction. This has resulted in inhibition of Hh signaling as well as antitumour activity in various animal models. In a transgenic mouse model of islet cell neoplasms, tumour volume was reduce by 95% in mice treated with sonidegib when compared with untreated mice. (2) Sonidegib (Erismodegib; LDE225; NVP-LDE225) is a potent, selective oral small-molecule antagonist of the SMO receptor, inhibiting the Hh signaling pathway [1] - Its mechanism of action involves binding to the transmembrane domain of SMO, preventing activation by Hh ligands and blocking downstream Gli transcription factor-mediated proliferation of Hh-dependent cancer cells [1][2] - The drug exhibits in vitro and in vivo efficacy against Hh pathway-dependent tumors, including BCC and CML [1][2] - It is clinically indicated for the treatment of locally advanced or metastatic basal cell carcinoma [1] - Drug-drug interactions: Co-administration with CYP3A4 inhibitors increases plasma concentrations of Sonidegib (Erismodegib; LDE225; NVP-LDE225), while CYP3A4 inducers decrease concentrations [1] |
| 分子式 |
C26H26F3N3O3
|
|---|---|
| 分子量 |
485.5
|
| 精确质量 |
485.192
|
| 元素分析 |
C, 64.32; H, 5.40; F, 11.74; N, 8.66; O, 9.89
|
| CAS号 |
956697-53-3
|
| 相关CAS号 |
Sonidegib diphosphate; 1218778-77-8
|
| PubChem CID |
24775005
|
| 外观&性状 |
White to light yellow solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
544.5±50.0 °C at 760 mmHg
|
| 闪点 |
283.1±30.1 °C
|
| 蒸汽压 |
0.0±1.5 mmHg at 25°C
|
| 折射率 |
1.569
|
| LogP |
5.43
|
| tPSA |
63.69
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
35
|
| 分子复杂度/Complexity |
691
|
| 定义原子立体中心数目 |
2
|
| SMILES |
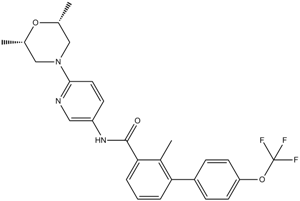
CC1C(C(=O)NC2C=NC(N3C[C@H](C)O[C@H](C)C3)=CC=2)=CC=CC=1C1C=CC(OC(F)(F)F)=CC=1
|
| InChi Key |
VZZJRYRQSPEMTK-CALCHBBNSA-N
|
| InChi Code |
InChI=1S/C26H26F3N3O3/c1-16-14-32(15-17(2)34-16)24-12-9-20(13-30-24)31-25(33)23-6-4-5-22(18(23)3)19-7-10-21(11-8-19)35-26(27,28)29/h4-13,16-17H,14-15H2,1-3H3,(H,31,33)/t16-,17+
|
| 化学名 |
N-[6-[(2S,6R)-2,6-dimethylmorpholin-4-yl]pyridin-3-yl]-2-methyl-3-[4-(trifluoromethoxy)phenyl]benzamide
|
| 别名 |
Sonidegib; LDE 225; NVP-LDE225; LDE-225; NVP LDE-225; LDE225; NVP LDE225; Erismodegib; trade name of Odomzo
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.15 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (5.15 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.15 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 2% DMSO+corn oil: 10 mg/mL 配方 5 中的溶解度: 2 mg/mL (4.12 mM) in 75% PEG 300 25% (5% dextrose in water) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0597 mL | 10.2987 mL | 20.5973 mL | |
| 5 mM | 0.4119 mL | 2.0597 mL | 4.1195 mL | |
| 10 mM | 0.2060 mL | 1.0299 mL | 2.0597 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Oral Hedgehog Inhibitors in the Treatment of Basal Cell Carcinoma in the Netherlands: a Prospective Registration Study
CTID: NCT05463757
Phase: Status: Recruiting
Date: 2024-05-22
 Antitumor activity in an orthotopic Ptch+/−p53−/−medulloblastoma allograft model in nude mice upon treatment with5mdiphosphate salt dosed at 40 mg/kg/day po bid or vehicle at equal dose volume.ACS Med Chem Lett. 2010 Jun 10; 1(3): 130–134. |
|---|
 Antitumor activity upon treatment with5mdiphosphate salt or vehicle in a Ptch+/−p53−/− medulloblastoma subcutaneous allograft model in nude mice.ACS Med Chem Lett. 2010 Jun 10; 1(3): 130–134. |
Gli1 mRNA inhibition (open circle), tumor PK (filled squares), and plasma PK (filled triangles) in Ptch+/−p53−/−medulloblastoma model after treatment with5m (Sonidegib, or erismodegib, LDE225, NVP-LDE225).ACS Med Chem Lett. 2010 Jun 10; 1(3): 130–134. |