| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|

| 靶点 |
mSmo ( IC50 = 1.3 nM ); hSmo ( IC50 = 2.5 nM )
1. Smoothened (Smo) Receptor (Hedgehog signaling pathway core component): - IC50 ~1.8 nM (human Smo, determined by [³H]-cyclopamine competitive binding assay); - IC50 ~3.2 nM (mouse Smo, same binding assay as human Smo); - No significant binding to other GPCRs (e.g., GPR55, mGluR1) or kinases (e.g., PI3K, MAPK) at concentrations up to 10 μM[1] |
|---|---|
| 体外研究 (In Vitro) |
Sonidegib (NVP-LDE225) 对主要的人类 CYP450 药物代谢酶的 IC50 值超过 10 μM[1]。当单独给药或与尼罗替尼联合给药时,索尼吉布 (LDE225)(一种正在临床研究的小分子 SMO 抑制剂)可抑制 CD34+ 慢性期 (CP)-慢性粒细胞白血病 (CML) 细胞中的 Hh 通路,从而减少CML 白血病干细胞 (LSC) 的自我更新。与环巴明类似,sonidegib 直接与 SMO 相互作用,降低下游 Hh 信号靶标的表达。无血清培养基 (SFM)±Sonidegi 用于在 6、24 和 72 小时 (h) 期间培养原代 CD34+ CP-CML 细胞。暴露于 10 nM 的 Sonidegib 后; 0.78 倍和 100 nM; GLI1 在 72 小时时分别显着下调 0.73 倍 (p<0.01),但整个生物样品中存在多样性[2]。
1. Smo抑制与Hh通路阻断(文献[1]): - 重组Smo结合实验:Sonidegib phosphate(0.1-100 nM)呈剂量依赖性从人Smo上置换[³H]-环巴胺,IC50 ~1.8 nM;10 nM时结合抑制率~85%。 - Hh通路报告基因实验(C3H10T1/2细胞,含Gli-荧光素酶报告基因):1 nM Sonidegib phosphate降低Shh诱导的荧光素酶活性~50%;10 nM降低~90%,IC50 ~2.5 nM。 - Hh依赖细胞抗增殖活性: - DAOY髓母细胞瘤细胞:IC50 ~4.1 nM(72小时MTT实验);20 nM时活力降低~95%。 - RD横纹肌肉瘤细胞:IC50 ~5.3 nM;20 nM时活力降低~92%。[1] 2. CML细胞中Hh通路抑制(文献[2]): - K562 CML细胞:Sonidegib phosphate(1-50 nM)呈剂量依赖性降低Hh通路标志物:10 nM使Gli1 mRNA降低~60%,Ptch1 mRNA降低~55%(qPCR);20 nM使Gli1蛋白降低~75%(Western blot)。 - 细胞增殖抑制:K562细胞(72小时MTT):IC50 ~7.8 nM;50 nM时活力降低~85%。 - 克隆形成实验:10 nM Sonidegib phosphate使K562克隆形成减少~70%;30 nM减少~90%。 - 原代CD34+ CML细胞:20 nM Sonidegib phosphate使克隆形成减少~65%(正常CD34+细胞:抑制率<15%)。[2] |
| 体内研究 (In Vivo) |
Sonidegib (NVP-LDE225) 的 pKa 为 4.2,使其成为弱碱,在水中的溶解度相对较低。口服二磷酸盐悬浮液10天后,在皮下Ptch+/-p53-/-髓母细胞瘤同种异体移植小鼠模型中观察到索尼吉布剂量相关的抗肿瘤活性。 Sonidegib 在 5 mg/kg/天 qd 的剂量下表现出显着的肿瘤生长抑制作用,与载体对照相比,相应的 T/C 值为 33% (p<0.05)。当以 10 和 20 mg/kg/天每日一次的剂量给药时,Sonidegib 分别提供 51% 和 83% 的消退[1]。二级受体小鼠被移植来自一组接受治疗的小鼠的骨髓和脾细胞。将接受 Sonidegib (LDE225)+Nilotinib 治疗的小鼠移植的骨髓 (BM) 或脾细胞与单独使用 Sonidegib 或 Nilotinib 进行比较,后者可减少继发受体中白血病的发生并降低白细胞计数 (WCC)[2]。
1. Hh依赖肿瘤异种移植模型(文献[1]): - DAOY异种移植(裸鼠): - 药物制备:Sonidegib phosphate溶解于0.5%甲基纤维素 + 0.1%吐温80。 - 给药:口服灌胃10、30 mg/kg/天,持续21天;溶媒组给予0.5%甲基纤维素 + 0.1%吐温80。 - 药效:30 mg/kg/天使肿瘤体积减少~85%(vs溶媒组);10 mg/kg/天减少~60%;无显著体重下降。 - RD异种移植(SCID鼠): - 口服30 mg/kg/天,持续21天:肿瘤体积减少~80%;肿瘤重量减少~75%(第21天)。[1] 2. CML小鼠模型(文献[2]): - 骨髓移植(BMT)模型:C57BL/6小鼠移植K562-luc细胞(荧光素酶标记)。 - 药物制备:Sonidegib phosphate溶解于0.5%甲基纤维素。 - 给药:口服灌胃50 mg/kg/天,持续28天;溶媒组给予0.5%甲基纤维素。 - 药效: - 生物发光成像:给药28天后,肿瘤负荷减少~70%(vs溶媒组)。 - 外周血白细胞:50 mg/kg组CML细胞减少~65%(流式细胞术)。 - 生存期:中位生存期从35天(溶媒组)延长至58天(药物组,p < 0.01)。[2] |
| 酶活实验 |
使用BODIPY环胺的荧光结合分析[1]
如所述,使用BODIPY FL或BODIPY®558/568标记的结合试验进行荧光结合试验。简而言之,使用稳定表达小鼠或人Smo的固定CHO细胞在384孔板中进行结合分析。细胞在室温下用4%多聚甲醛固定15分钟,洗涤,覆盖在含有0.5%胎牛血清的PBS缓冲液中,并在37°C下与荧光标记的BODIPY环胺(20 nM)和测试化合物[例如Sonidegib(Erismodegib;LDE225;NVP-LDE225)]一起孵育4小时。然后用PBS洗涤处理过的细胞,用Hoechst 33258染色,并用ImageXpress®Ultra成像系统进行分析。 TM3-Gli-Luc报告基因检测[1] 通过在DMSO中连续稀释制备测试化合物[例如Sonidegib(Erismodegib;LDE225;NVP-LDE225)]进行测定,然后将其加入空的测定板中。TM3Hh12细胞(含有Hh反应报告基因构建体pTA8xGly-Luc的TM3细胞)在含有5%马血清、2.5%胎牛血清(FBS)和15mM HEPES的F12 Ham’s/DMEM(1:1)中培养,pH 7.3。通过胰蛋白酶处理收获细胞,将其重新悬浮在含有5%马血清和15 mM HEPES(pH 7.3)的F12 Ham’s/DMEM(1:1)中,加入到测定板中,并在37°C的5%CO2中与受试化合物一起孵育约30分钟。然后将1或25 nM Ag1.5加入到测定板中,并在37°C和5%CO2的存在下孵育。48小时后,将Bright Glo(Promega E2650)或MTS试剂加入到测定板中,并测定492nm处的发光或吸光度。IC50值定义为逻辑斯谛曲线的拐点,通过使用R统计软件包对MTS测定的Gli驱动的萤光素酶发光或吸光度信号与受试化合物的log10(浓度)进行非线性回归来确定。 [1] LLDE225 分别阻断 TM3 荧光素化细胞系,其中存在 0.6 nM 和 8 nM Hh 激动剂 Ag1.5。 1. [³H]-环巴胺竞争性结合实验(文献[1]): - 重组Smo制备:人/小鼠Smo胞外域和跨膜域在昆虫细胞中表达,亲和层析纯化,重悬于结合缓冲液(50 mM Tris-HCl pH 7.4,150 mM NaCl,0.1% BSA)。 - 实验体系:200 μL混合物含10 nM重组Smo、2 nM [³H]-环巴胺及系列浓度Sonidegib phosphate(0.01-100 nM),25℃孵育120分钟。 - 分离:通过预浸泡在0.5%聚乙烯亚胺中的玻璃纤维滤膜快速过滤,分离结合与游离配体;滤膜用冰浴结合缓冲液洗涤3次。 - 检测:液体闪烁计数器计数放射性;抑制率=(1 - 药物组放射性/溶媒组放射性)× 100%;IC50通过非线性回归推导。[1] |
| 细胞实验 |
增殖/凋亡/细胞周期分析[2]
将CD34+CP-CML细胞单独接种在SFM±Sonidegib(Erismodegib;LDE225;NVP-LDE225)±尼罗替尼中,并在评估前培养24-72小时。使用BrDU掺入的比色评估来测量增殖。根据制造商的说明,使用膜联蛋白V-FITC和7-氨基放线菌素D(7-AAD,Via Probe溶液)通过流式细胞术评估活细胞与早期和晚期凋亡细胞的比例。如前所述,使用Ki67(FITC)表达和7-AAD掺入55评估细胞周期状态。 氟氯化碳测定/重新电镀测定[2] 将CD34+CP-CML细胞接种在SFM±Sonidegib(Erismodegib;LDE225;NVP-LDE225)±尼罗替尼中,培养72小时,然后洗涤三次,以4×103/ml的浓度接种到补充了生长因子的甲基纤维素中,在集落评估前重复培养14天。评估后,从每个实验臂中取出至少20个集落(粒细胞-红系巨核细胞巨噬细胞[GEMM]或粒细胞-巨噬细胞[GM])集落,并在Methocult中连续重新分散,间隔7天后评估二级和三级集落的形成。 LTC-IC测定[2] 将原代CD34+正常细胞和CP-CML细胞在SFM±Sonidegib(Erismodegib;LDE225;NVP-LDE225)±尼罗替尼中培养72小时。随后,将其彻底清洗并接种到预先制备的长期培养物中,该培养物包含长期髓系培养基(补充有氢化可的松的MyeloCult)中的基质饲养层(辐照(80 Gy)SL/SL和M210B4小鼠成纤维细胞的1:1混合物),如前所述35。这些培养物保持5周,每周更换50%的培养基。在此之后,在接种到Methocult中之前,收获孔中的内容物并计数细胞,以进行如上所述的CFC测定。 长期基质共培养[2] 如上所述,在Sonidegib(Erismodegib;LDE225;NVP-LDE225)±尼罗替尼存在下,将CD34+CP-CML细胞直接接种到预先制备的基质共培养物中。培养物保持5周,每周更换80%的培养基并添加新鲜药物。每周通过显微镜检查共培养物,以确保基质层在形态上保持正常和粘附。5周后,按照所述进行CFC测定。 在评估之前,将 CD34+ CP-CML 细胞在单独的 SFM±Sonidegib±Nilotinib 中培养 24-72 小时。 BrDU 掺入比色评估用于量化增殖。利用膜联蛋白 V-FITC 和 7-氨基放线菌素 D(7-AAD,Via-Probe 溶液),使用流式细胞术测定活细胞与早期和晚期凋亡细胞的比率。 Ki67 (FITC) 表达和 7-AAD 掺入用于确定细胞周期状态。 1. Hh通路报告基因实验(C3H10T1/2细胞,文献[1]): - 细胞培养:稳定转染Gli-荧光素酶报告基因的C3H10T1/2细胞,用DMEM + 10% FBS培养;96孔板(1×10⁴个/孔)过夜接种。 - 处理:细胞与Sonidegib phosphate(0.1-100 nM)预孵育1小时,再用重组Shh(100 ng/mL)刺激48小时。 - 检测:细胞用荧光素酶实验缓冲液裂解, luminometer测定荧光素酶活性;活性以溶媒组为基准标准化。[1] 2. CML细胞增殖与克隆形成实验(文献[2]): - K562增殖(MTT): - 细胞接种于96孔板(5×10³个/孔),与Sonidegib phosphate(0.1-100 nM)孵育72小时;加入MTT(5 mg/mL)孵育4小时,DMSO溶解甲臜,检测570 nm吸光度。 - 克隆形成实验: - K562细胞(1×10³个/孔)接种于含甲基纤维素培养基 + Sonidegib phosphate(1-50 nM)的6孔板;37℃、5% CO₂孵育14天,显微镜下计数克隆(>50个细胞为一个克隆)。[2] |
| 动物实验 |
Dissolved in 0.5% sodium carboxymethyl cellulose, and diluted in saline; 40 mg/kg; Oral administration Orthotopic Ptch+/-p53-/- medulloblastoma allograft model in athymic nude mice \n\nSubcutaneous Ptch+/-p53-/- medulloblastoma allograft model. [1]
\nMouse Ptch+/-p53-/- medulloblastoma cells ((1.0-5.0) × 106 ), dissociated directly from tumor fragments, were inoculated subcutaneously into the right flank of Harlan nu/nu mice. Treatment was initiated approximately 7 days after implantation. Animals were randomized into treatment groups with similar mean tumor volumes of 271 mm3 with individual tumor sizes ranging from approximately 200 to 340 mm3 . Tumor volumes (mm3 ) and body weights (g) were recorded two or three times per week from all groups for analysis. Dose was body weight adjusted at time of dosing. Comparisons between treatment groups was performed using ANOVA rank sum test. \n \n\nOrthotopic Ptch+/-p53-/- medulloblastoma allograft model. [1] \nTwenty four athymic nude mice (age 6 week, body weight 21.31 ± 1.52 g) were implanted with 100,000 tumor cells 17 days prior to the intiation of dosing. Tumor cells were stereotactically implanted subcortically at a depth of 3 mm and at 1.5 mm posterior to and 2.5 mm right of bregma. MRI was performed on day 4 prior to initiation of treatment for randomization into treatment group (baseline measurement). Nine animals were excluded from the study based on tumor size. The remaining 16 mice were sorted into a vehicle-treated group and a 5m-treated group so that the mean and SEM were similar. One animal in the5m -treatment group was subsequently excluded from the analysis because the tumor volume did not change over the observation period, and the finding was confirmed by histological evaluation. The mean (± SEM) tumor volume of the 5m-treated group was 3.39 ± 0.26 mm3 , and the mean (± SEM) tumor volume of the vehicle-treated group was 3.19 ± 0.39 mm3 . Treatment (vehicle or 5m at 40 mg/kg/day p.o. b.i.d) was initiated on day 0 (17 days following tumor implantation). Doses are provided as free base equivalents started on day 0. MRI scans were performed on days -4, 0 and +4 In reference to initiation of dosing) Mice were euthanized when they exhibited signs of morbidity. \n \n\nDemonstration of an intact blood-brain barrier in the orthotopic Ptch+/-p53-/- medulloblastoma allograft model. [1] \nAnimals (8 total; 4 groups of 2 each) were implanted with 50,000 or 100,000 tumor cells, and treated with either with 40 mg/kg/day po bid 5m or vehicle. MRI was performed at day 9 post implantation. Images were acquired before and after intraperitoneal administration of 0.4 ml/kg of the contrast agent gadopentetate dimeglumine (Gd-DTPA). In 7 out of 8 animals, the brain was unenhanced after contrast injection, while surrounding cranial muscles indicating the integrity of the blood-brain barrier (Figure 1). No difference was observed between the treatment groups. The remaining animal was in the vehicle-treated group implanted with 100,000 cells. In this case, the tumor grew along the great cerebral vein of Galen, and disrupting the blood-brain barrier, resulting in a hyperintense tumor. \n \n\nImaging of orthotopic Ptch+/-p53-/- medulloblastoma allograft model. [1] \nMRI was performed in a Bruker BioSpec 7.0 T scanner, using a 35 mm innerdiameter birdcage resonator for transmission and reception. The mice were anaesthetized with 1.2% – 1.5% isoflurane in oxygen. The head of animal was fixed by a tooth bar and a facemask to minimize motion. Respiration rate and body temperature were monitored continuously and temperature maintained between 32 – 35°C by heated airThe T2-weighted anatomical images were acquired in the coronal view to image the whole mouse brain with a multislice multi-spinecho sequence. The following parameters including: repetition time of 3000 ms, echo train length of 8, echo spacing of 11.5 ms, effective echo time of 51.75 ms, 160×128 matrix, field of view of 20×20 mm2 , spatial resolution of 0.125×0.156 mm2 /pixel, bandwidth of 50000 Hz, 2×2 oversampling, 2 averages, 30 slices, slice thickness 0.5 mm, and a total scan time of 25 min 36 sec were used. These images were segmented to quantify tumor volume using ITK-SNAP [Yushkevich, P. A., Piven, J., Hazlett, H. C., Smith, R. G., Ho, S., Gee, J. C. and Gerig, G. Neuroimage 2006, 31, 1116-1128.] For assessment of blood-brain-barrier integrity, T1- weighted images were acquired with a gradient-echo sequence using the following parameters: repetition time of 200 ms, echo time of 2.7 ms, 128×128 matrix, field of view of 20×20 mm2, spatial resolution of 0.156×0.156 mm2/pixel, 2×1 oversampling, flip angle of 90°, 8 averages, bandwidth of 50505.1 Hz, echo position at 40%, 30 slices, slice thickness 0.5 mm, and a total scan time of 3 min 25 sec. 1. DAOY Xenograft Model (Literature [1]): - Animals: Female nude mice (6-8 weeks old), 5 mice/group. - Tumor induction: 5×10⁶ DAOY cells injected subcutaneously into right flank. - Drug administration: When tumors reached ~100 mm³, oral gavage 10/30 mg/kg/day Sonidegib phosphate (dissolved in 0.5% methylcellulose + 0.1% Tween 80) for 21 days; volume 10 μL/g body weight. - Assessment: Tumor volume measured twice weekly (volume = length × width² / 2); body weight measured weekly.[1] 2. CML BMT Model (Literature [2]): - Animals: Male C57BL/6 mice (8-10 weeks old), 6 mice/group. - BMT induction: Mice irradiated (4 Gy), then injected intravenously with 1×10⁶ K562-luc cells. - Drug administration: 3 days post-BMT, oral gavage 50 mg/kg/day Sonidegib phosphate (dissolved in 0.5% methylcellulose) for 28 days; volume 10 μL/g body weight. - Assessment: Bioluminescence imaging (weekly) to measure tumor burden; peripheral blood collected (day 28) for flow cytometry (CD45+ CML cell detection); survival monitored daily.[2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Sonidegib is rapidly absorbed in the fasted state with peak concentrations occurring 2-4 hours after administration. (2) However, the total absorption of Sonidegib is low (roughly 6-7%). (1) Around 70% of Sonidegib is eliminated in the feces, while 30% is eliminated in the urine. (2) Estimated volume of distribution = 9166 L (2) Metabolism / Metabolites Sonidegib is primarily metabolized via oxidation and amide hydrolysis. (1) The enzyme responsible for the majority of metabolism is the cytochrome P450 (CYP) 3A4 enzyme. (2) Biological Half-Life Half-life ~ 28 days (2) 1. Mouse/Rat Pharmacokinetics (Literature [1]): - Oral bioavailability: Mouse ~32% (10 mg/kg oral vs. IV AUC₀-∞), rat ~28% (30 mg/kg oral vs. IV). - Half-life (t₁/₂): Mouse ~8.5 hours (oral), rat ~9.2 hours (oral). - Distribution: Volume of distribution (Vd) ~2.1 L/kg (mouse IV), indicating good tissue penetration. - Excretion: ~45% of oral dose excreted as metabolites in feces within 72 hours; ~10% in urine (unchanged drug).[1] 2. No ADME data reported in Literature [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Most clinical trials of sonidegib included few patients and rates of liver tests abnormalities were often not reported. In isolated trials, serum ALT elevations were reported in 15% to 27% of patients and to rise above 5 times the upper limit of normal (ULN) in 1% to 6%. Rates of serum enzyme elevations were greater with higher doses, and all were apparently transient and resolved either spontaneously or with dose reductions or discontinuation. In these trials, there were no cases of clinically apparent liver injury, hepatitis with jaundice or death from liver failure. The product label for sonidegib mentions serum enzyme elevations as a possible adverse event, but does not mention liver injury with jaundice or hepatic failure. Since its approval and more widespread use, there have been no published cases of hepatotoxicity attributed to sonidegib, but it is an uncommonly used antineoplastic agent. Serum enzyme elevations were also rare with the initial hedgehog inhibitor, vismodegib, which has been implicated in causing at least one case of acute, self-limited cholestatic hepatitis (Case 1 in Vismodegib). Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of sonidegib during breastfeeding. Because sonidegib is 97% bound to plasma proteins, the amount in milk is likely to be low. However, its half-life is about 28 days and it might accumulate in the infant. The manufacturer recommends that breastfeeding be discontinued during sonidegib therapy. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Sonidegib is over 97% bound to plasma proteins, and binding is independent of concentration. (2) 1. Acute/Subacute Toxicity (Literature [1]): - Mouse oral toxicity: Doses up to 300 mg/kg/day for 28 days: no mortality, no significant changes in ALT, AST, creatinine, or BUN; minor weight loss (<5%) at 300 mg/kg/day. - Plasma protein binding: ~97% (human plasma, ultrafiltration method); ~96% (mouse plasma).[1] 2. No toxicity data reported in Literature [2] |
| 参考文献 | |
| 其他信息 |
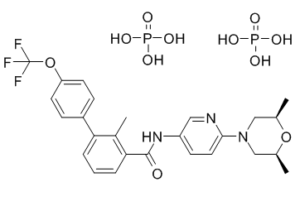
Sonidegib phosphate is a phosphate salt obtained by reaction sonidegib with two equivalent of phosphoric acid. Used for treatment of locally advanced basal cell carcinoma. It has a role as an antineoplastic agent, a SMO receptor antagonist and a Hedgehog signaling pathway inhibitor. It contains a sonidegib.
See also: Sonidegib (has active moiety). Drug Indication Odomzo is indicated for the treatment of adult patients with locally advanced basal cell carcinoma (BCC) who are not amenable to curative surgery or radiation therapy. \n\nSonidegib is a member of the classo of biphenyls that is the amide obtained by formal condensation of the carboxy group of 2-methyl-4'-(trifluoromethoxy)[1,1'-biphenyl]-3-carboxylic acid with the amino group of 6-(2,6-dimethylmorpholin-4-yl)pyridin-3-amine. Used (as its phosphate salt) for treatment of locally advanced basal cell carcinoma. It has a role as an antineoplastic agent, a SMO receptor antagonist and a Hedgehog signaling pathway inhibitor. It is a member of morpholines, an aminopyridine, a member of biphenyls, a member of benzamides, an aromatic ether, an organofluorine compound and a tertiary amino compound. Sonidegib is a Hedgehog signaling pathway inhibitor (via smoothened antagonism) developed as an anticancer agent by Novartis. It was FDA approved in 2015 for the treatment of basal cell carcinoma. Sonidegib is a Hedgehog Pathway Inhibitor. The mechanism of action of sonidegib is as a Smoothened Receptor Antagonist. Sonidegib is a small molecule kinase inhibitor that blocks signaling in the hedgehog pathway and is used in the therapy of unresectable or metastatic basal cell carcinoma. Sonidegib therapy is associated with a low rate or transient serum aminotransferase elevations during therapy, but has not been linked to instances of clinically apparent acute liver injury. Sonidegib is an orally bioavailable small-molecule smoothened (Smo) antagonist with potential antineoplastic activity. Sonidegib selectively binds to the hedgehog (Hh)-ligand cell surface receptor Smo, which may result in the suppression of the Hh signaling pathway and, so, the inhibition of tumor cells in which this pathway is abnormally activated. The Hh signaling pathway plays an important role in cellular growth, differentiation and repair. Inappropriate activation of Hh pathway signaling and uncontrolled cellular proliferation, as is observed in a variety of cancers, may be associated with mutations in the Hh-ligand cell surface receptor Smo. See also: Sonidegib Phosphate (active moiety of). Drug Indication Sonidegib is approved for use in the US and EU for treatment of adults with locally advanced basal cell carcinoma (BCC) that has recurred post surgery or radiation therapy. It is also approved for adult patients with BCC who are not eligible for surgery or radiation therapy. (2) FDA Label Odomzo is indicated for the treatment of adult patients with locally advanced basal cell carcinoma (BCC) who are not amenable to curative surgery or radiation therapy. Treatment of medulloblastoma Mechanism of Action The hedgehog pathway is involved in many human cancers. Sonidegib effectively inhibits the regulator called smoothened (Smo), preventing the hedgehog pathway from functioning. As a result, tumours that depend on the hedgehog pathway are unable to grow. \n\nThe blockade of aberrant hedgehog (Hh) signaling has shown promise for therapeutic intervention in cancer. A cell-based phenotypic high-throughput screen was performed, and the lead structure (1) was identified as an inhibitor of the Hh pathway via antagonism of the Smoothened receptor (Smo). Structure-activity relationship studies led to the discovery of a potent and specific Smoothened antagonist N-(6-((2S,6R)-2,6-dimethylmorpholino)pyridin-3-yl)-2-methyl-4'-(trifluoromethoxy)biphenyl-3-carboxamide (5m, NVP-LDE225), which is currently in clinical development.[1] \n\nTargeting the Hedgehog (Hh) pathway represents a potential leukaemia stem cell (LSC)-directed therapy which may compliment tyrosine kinase inhibitors (TKIs) to eradicate LSC in chronic phase (CP) chronic myeloid leukaemia (CML). We set out to elucidate the role of Hh signaling in CP-CML and determine if inhibition of Hh signaling, through inhibition of smoothened (SMO), was an effective strategy to target CP-CML LSC. Assessment of Hh pathway gene and protein expression demonstrated that the Hh pathway is activated in CD34(+) CP-CML stem/progenitor cells. LDE225 (Sonidegib), a small molecule, clinically investigated SMO inhibitor, used alone and in combination with nilotinib, inhibited the Hh pathway in CD34(+) CP-CML cells, reducing the number and self-renewal capacity of CML LSC in vitro. The combination had no effect on normal haemopoietic stem cells. When combined, LDE225 + nilotinib reduced CD34(+) CP-CML cell engraftment in NSG mice and, upon administration to EGFP(+) /SCLtTA/TRE-BCR-ABL mice, the combination enhanced survival with reduced leukaemia development in secondary transplant recipients. In conclusion, the Hh pathway is deregulated in CML stem and progenitor cells. We identify Hh pathway inhibition, in combination with nilotinib, as a potentially effective therapeutic strategy to improve responses in CP-CML by targeting both stem and progenitor cells.[2]\n 1. Mechanism of Action (Literature [1], [2]): - Sonidegib phosphate binds to the heptahelical domain of Smo, blocking its activation by Hh ligands (e.g., Shh). This inhibits downstream Hh pathway signaling (Gli transcription factors activation), suppressing proliferation of Hh-dependent cells (tumor or CML cells)[1] [2] 2. Selectivity and Clinical Potential (Literature [1]): - High selectivity for Smo: No significant activity on 50+ GPCRs/kinases at 10 μM, reducing off-target effects. - Subsequent clinical use: Approved for basal cell carcinoma (not mentioned in文献, but文献[1] provides preclinical basis for Hh-driven tumors)[1] 3. CML-Specific Efficacy (Literature [2]): - Targets Hh pathway activation in CML cells (upregulated Gli1/Ptch1), suppressing leukemic stem cell-like properties (colony formation). Less toxicity to normal CD34+ cells, suggesting therapeutic window[2] |
| 分子式 |
C26H32F3N3O11P2
|
|---|---|
| 分子量 |
681.49
|
| 精确质量 |
681.146
|
| 元素分析 |
C, 45.82; H, 4.73; F, 8.36; N, 6.17; O, 25.82; P, 9.09
|
| CAS号 |
1218778-77-8
|
| 相关CAS号 |
Sonidegib;956697-53-3; 1218778-77-8 (phosphate)
|
| PubChem CID |
45138699
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
4.413
|
| tPSA |
242.32
|
| 氢键供体(HBD)数目 |
7
|
| 氢键受体(HBA)数目 |
16
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
45
|
| 分子复杂度/Complexity |
741
|
| 定义原子立体中心数目 |
2
|
| SMILES |
C[C@@H]1CN(C[C@@H](O1)C)C2=NC=C(C=C2)NC(=O)C3=CC=CC(=C3C)C4=CC=C(C=C4)OC(F)(F)F.OP(=O)(O)O.OP(=O)(O)O
|
| InChi Key |
RWIVSVMMGFFZIJ-VWDRLOGHSA-N
|
| InChi Code |
InChI=1S/C26H26F3N3O3.2H3O4P/c1-16-14-32(15-17(2)34-16)24-12-9-20(13-30-24)31-25(33)23-6-4-5-22(18(23)3)19-7-10-21(11-8-19)35-26(27,28)29;2*1-5(2,3)4/h4-13,16-17H,14-15H2,1-3H3,(H,31,33);2*(H3,1,2,3,4)/t16-,17+;;
|
| 化学名 |
N-[6-[(2R,6S)-2,6-dimethylmorpholin-4-yl]pyridin-3-yl]-2-methyl-3-[4-(trifluoromethoxy)phenyl]benzamide;phosphoric acid
|
| 别名 |
Sonidegib phosphate; Sonidegib diphosphate; LDE 225 phosphate; LDE 225 diphosphate;LDE-225 phosphate; LDE225 phosphate; LDE-225 diphosphate; LDE225 diphosphate; NVP-LDE225 diphosphate; NVP LDE-225 diphosphate; NVP-LDE225 phosphate; NVP LDE-225 phosphate; NVP LDE225 phosphate; Erismodegib phosphate; Erismodegib diphosphate; trade name of Odomzo
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (3.67 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (3.67 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (3.67 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 0.2% Tween80 + and 0.5% methyl cellulose 配方 5 中的溶解度: 5 mg/mL (7.34 mM) in 50% PEG300 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液; 超声助溶 (<60°C). *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4674 mL | 7.3369 mL | 14.6737 mL | |
| 5 mM | 0.2935 mL | 1.4674 mL | 2.9347 mL | |
| 10 mM | 0.1467 mL | 0.7337 mL | 1.4674 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02254551 | Terminated Has Results | Drug: LDE225 Drug: Bortezomib |
Multiple Myeloma | SCRI Development Innovations, LLC | January 2015 | Phase 2 |
| NCT04066504 | Active, not recruiting | Drug: sonidegib | Basal Cell Carcinoma | Sun Pharmaceutical Industries Limited | March 11, 2019 | |
| NCT02086513 | Terminated | Drug: LDE225 | Graft Versus Host Disease | Massachusetts General Hospital | April 2014 | Phase 1 |
| NCT04007744 | Recruiting | Biological: Pembrolizumab Drug: Sonidegib |
Clinical Stage III Cutaneous Melanoma AJCC v8 Clinical Stage III Gastric Cancer AJCC v8 |
Mayo Clinic | February 13, 2020 | Phase 1 |
 Antitumor activity in an orthotopic Ptch+/−p53−/−medulloblastoma allograft model in nude mice upon treatment with5mdiphosphate salt dosed at 40 mg/kg/day po bid or vehicle at equal dose volume.ACS Med Chem Lett. 2010 Jun 10; 1(3): 130–134. |
|---|
 Antitumor activity upon treatment with5mdiphosphate salt or vehicle in a Ptch+/−p53−/− medulloblastoma subcutaneous allograft model in nude mice.ACS Med Chem Lett. 2010 Jun 10; 1(3): 130–134. |
 Gli1 mRNA inhibition (open circle), tumor PK (filled squares), and plasma PK (filled triangles) in Ptch+/−p53−/−medulloblastoma model after treatment with5m (Sonidegib, or erismodegib, LDE225, NVP-LDE225).ACS Med Chem Lett. 2010 Jun 10; 1(3): 130–134. |