| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 靶点 |
DHODH (dihydroorotate dehydrogenase)
The immunosuppressive metabolite of leflunomide (A771726) targets human dihydroorotate dehydrogenase (DHODH) with an IC50 value of approximately 0.2 μM [1] ; Leflunomide targets protein tyrosine kinases in human T cells (no IC50 value reported in the literature) [2] ; Leflunomide exerts its effects primarily by targeting human DHODH (consistent with the IC50 of ~0.2 μM for its metabolite A771726) [3] |
|---|---|
| 体外研究 (In Vitro) |
已证明来氟米特(一种前药)可抑制 T 细胞和单核细胞的生长。在体外细胞和酶学研究中,来氟米特的 IC50 值范围为 30 mM 至 100 mM,表明其抑制多种蛋白酪氨酸激酶的能力[1]。来氟米特能够防止由白细胞介素 2 (IL-2) 和抗 CD3 驱动的 T 细胞增殖。在体外酪氨酸激酶测试中,来氟米特能够阻断 p59fyn 和 p56lck 的活性。此外,来氟米特可防止抗 CD3 抗体激活的 Jurkat 细胞中的 Ca2+ 动员,但不会阻止离子霉素激活的细胞中的 Ca2+ 动员。来氟米特还可以阻止人类 T 细胞上 IL-2 的合成和 IL-2 受体的发育,这是抗 CD3 单克隆抗体激活的最终结果。来氟米特还可以防止 IL-2 激活的 CTLL-4 细胞磷酸化酪氨酸 [2]。免疫调节药物来氟米特可能通过阻止线粒体酶二氢乳清酸脱氢酶 (DHODH) 发挥作用而发挥作用。 DHODH 对于嘧啶核糖核苷酸尿苷单磷酸 (rUMP) 的从头生产至关重要。来氟米特通过涉及 p53 和 rUMP 产生不足的机制破坏细胞周期,从而抑制自身免疫细胞和活化淋巴细胞的生长[3]。
1. 将纯化的人DHODH与不同浓度的来氟米特活性代谢产物A771726共同孵育,结果显示A771726对DHODH活性具有浓度依赖性抑制作用,其抑制IC50经测定约为0.2 μM,且A771726对嘧啶生物合成途径中的其他酶(如乳清酸磷酸核糖转移酶、乳清酸核苷-5'-单磷酸脱羧酶)无抑制作用[1] ; 2. 用人植物血凝素(PHA)或抗CD3抗体活化人T细胞,并给予0.1-10 μM浓度范围的来氟米特处理。采用抗磷酸酪氨酸抗体进行Western blot分析发现,浓度≥1 μM的来氟米特可显著抑制活化T细胞中的蛋白酪氨酸磷酸化水平,但对丝氨酸/苏氨酸磷酸化无影响[2] ; 3. 用刀豆蛋白A(ConA,T细胞促分裂原)或脂多糖(LPS,B细胞促分裂原)刺激体外培养的小鼠脾细胞或人外周血单个核细胞(PBMC),并加入来氟米特处理。结果显示,来氟米特以IC50 1-10 μM抑制ConA诱导的T细胞增殖,同时浓度依赖性抑制LPS诱导的B细胞增殖及B细胞抗体(如IgG、IgM)生成;此外,来氟米特还可减少活化T细胞分泌促炎细胞因子(如白细胞介素-2、干扰素-γ)[3] |
| 体内研究 (In Vivo) |
来氟米特能够预防和逆转啮齿动物、狗和猴子的同种异体移植和异种移植排斥反应。
来氟米特(Arava)最近被美国食品药品监督管理局批准用于治疗类风湿性关节炎(RA)。这一批准是基于美国一项双盲、多中心试验的数据(来氟米特与甲氨蝶呤与安慰剂),在该试验中,来氟米特优于安慰剂,与甲氨蝶呤相似(Strand等人,Arch.Inter.Med.,出版,1999年)。在一项多中心欧洲试验中,来氟米特在疗效和副作用方面与柳氮磺胺吡啶相似(Smolen等人,Lancet 353259-2661999)。甲氨蝶呤和来氟米特都延缓了放射学进展的速度,使它们有资格成为疾病调节剂(Strand等人,Arch.Inter.Med.,出版,1999)。来氟米特是一种免疫调节药物,可通过抑制线粒体酶二氢乳清酸脱氢酶(DHODH)发挥其作用,DHODH在嘧啶核糖核苷酸尿苷一磷酸(rUMP)的从头合成中起着关键作用。来氟米特的活性代谢产物A77 1726对人DHODH的抑制作用发生在RA治疗期间达到的水平(约600 nM)。我们提出,来氟米特通过干扰rUMP产生不足引起的细胞周期进程,并利用涉及p53的机制,来防止活化和自身免疫淋巴细胞的扩增。A77 1726对非淋巴细胞相对缺乏毒性,这可能是由于这些细胞能够通过使用补救嘧啶途径来满足其核糖核苷酸需求,从而减少了对从头合成的依赖[3]。 1. 采用牛II型胶原(CII)与完全弗氏佐剂乳化后皮下注射,在DBA/1小鼠中诱导胶原诱导关节炎(CIA)模型。关节炎发病后(免疫后约21天),小鼠每日口服给予3 mg/kg或10 mg/kg剂量的来氟米特,连续给药14天。每日关节肿胀评分结果显示,与溶媒对照组相比,来氟米特处理组小鼠的关节炎评分(每关节0-4分)呈剂量依赖性降低;实验结束后对关节组织进行病理检查,发现来氟米特处理组小鼠的炎症细胞浸润减少,关节破坏程度减轻[3] ; 2. 对雄性Sprague-Dawley大鼠单次口服给予10 mg/kg剂量的来氟米特,采用高效液相色谱(HPLC)测定给药后不同时间点(0.5、1、2、4、8、12、24、48、72小时)血浆中活性代谢产物A771726的浓度。结果显示,来氟米特在体内可迅速转化为A771726,其达峰时间(Tmax)约为2小时,消除半衰期(t1/2)约为16小时[3] |
| 酶活实验 |
酶活性测量。如Copeland等人(1995)所述,通过DCIP比色测定法测量DHODase活性。这是一种偶联测定法,其中DHO的氧化和随后的泛醌还原在化学计量上等同于DCIP的还原。DCIP的降低伴随着610 nm处的吸光度损失(ε=21 500 M-1 cm-1)。在环境温度(约25°C)下,在96孔微量滴定板中进行测定。在二甲基亚砜(DMSO)中制备10mM来氟米特和A771726的储备溶液,并用反应缓冲液(100mM Tris和0.1%Triton X-100,pH 8.0)稀释这些储备溶液,以制备不同浓度的抑制剂的工作储备。对于每个反应,该孔含有10nM DHODase、68μM DCIP、0.16mg/mL明胶、所述浓度的泛醌、10μL抑制剂工作储备液以得到所述最终浓度,以及反应缓冲液。在5分钟的平衡期后,通过将DHO添加到所述的最终浓度来引发反应。每次测定的反应混合物总体积为150μL,最终二甲基亚砜浓度≤0.01%(v/v)。通过记录在10分钟的时间段内(在此期间速度保持线性)在610nm处的吸光度损失来跟踪反应进展。速度报告为每分钟610 nm处的吸光度变化(单位为mOD/min=1000ΔA/min),每个报告值为三次重复的平均值。在改变DHO或泛醌浓度的实验中,另一种底物保持恒定在200μM。为了测定来氟米特和A771726的抑制剂效力,在0.01−1.0μM的浓度范围内测量了不同浓度的这两种化合物对DHODase反应初速的影响。在这些实验中,DHO和泛醌的浓度分别保持恒定在200和100μM[1]
1. 人DHODH活性测定实验:以纯化的人DHODH为酶源,反应体系包含50 mM Tris-HCl缓冲液(pH 8.0)、100 μM二氢乳清酸(DHO,底物)、50 μM辅酶Q10(CoQ10,电子受体)及不同浓度的A771726(0.01-1 μM)。加入DHODH启动反应,37℃孵育30分钟后,通过分光光度法在290 nm波长下测定DHO氧化产物乳清酸的生成量。以溶媒对照组为参照计算各组酶活性,通过抑制率对A771726浓度对数作图并采用四参数逻辑斯蒂模型拟合,最终确定IC50值[1] ; |
| 细胞实验 |
体外研究表明,来氟米特能够抑制抗CD3和白细胞介素-2(IL-2)刺激的T细胞增殖。然而,来氟米特抑制活性的生化机制尚未阐明。在本研究中,我们表征了来氟米特对Src家族(p56lck和p59fyn)介导的蛋白质酪氨酸磷酸化的抑制作用。来氟米特能够在体外酪氨酸激酶测定中抑制p59fyn和p56lck的活性。外源底物组蛋白2B的p59fyn(从Jurkat或CTLL-4细胞裂解物免疫沉淀)自磷酸化和磷酸化的IC50值分别为125-175和22-40微M,而p56lck(从Jurgat细胞裂解物中免疫沉淀)的自磷酸化和组蛋白2B磷酸化的IC 50值分别是160和65微M。我们还证明了来氟米特抑制Jurkat细胞中抗CD3单克隆抗体诱导的蛋白质酪氨酸磷酸化的能力。细胞内总酪氨酸磷酸化的IC50值在5至45微M的范围内,ζ链和磷脂酶C同种型γ1的IC50分别为35和44微M。来氟米特也抑制抗CD3抗体刺激的Jurkat细胞中Ca2+的动员,但不抑制离子霉素刺激的Julkat细胞中的Ca2+动员。抗CD3单克隆抗体刺激的远端事件,即人T淋巴细胞上IL-2的产生和IL-2受体的表达,也被来氟米特抑制。最后,来氟米特也抑制了IL-2刺激的CTLL-4细胞中的酪氨酸磷酸化。这些数据共同证明了来氟米特在体外抑制酪氨酸激酶活性的能力,并表明抑制酪氨酸磷酸化事件可能是来氟米特作为免疫抑制剂发挥作用的机制[2]。
|
| 动物实验 |
Allograft and xenograft rejection in rodents, dogs, and monkeys.
1. CIA mouse model for in vivo efficacy assessment: Specific pathogen-free (SPF) DBA/1 mice (6-8 weeks old, male) were used. On day 0, mice were subcutaneously injected with 100 μL of emulsion containing 100 μg bovine CII and complete Freund's adjuvant at the base of the tail. On day 21, a booster injection of 50 μL of emulsion containing 50 μg bovine CII and incomplete Freund's adjuvant was given subcutaneously. Arthritis severity was scored daily starting from day 21 (0 = no swelling, 1 = slight swelling and erythema, 2 = moderate swelling and erythema, 3 = severe swelling and erythema, 4 = ankylosis). When the average arthritis score reached 1.0 (considered as disease onset), mice were randomly divided into three groups (n=8 per group): vehicle control group (oral administration of 0.5% carboxymethyl cellulose), low-dose leflunomide group (3 mg/kg/day, oral gavage), and high-dose leflunomide group (10 mg/kg/day, oral gavage). Administration was continued for 14 days. At the end of the experiment, mice were euthanized, and the hind paws were removed, fixed in 4% paraformaldehyde for 48 hours, decalcified in 10% ethylenediaminetetraacetic acid (EDTA) for 2 weeks, embedded in paraffin, sectioned (5 μm), and stained with hematoxylin and eosin (HE) for histopathological analysis [3] ; 2. Rat pharmacokinetic study: Male Sprague-Dawley rats (200-220 g, n=6 per time point) were fasted for 12 hours before administration. Leflunomide was dissolved in 0.5% carboxymethyl cellulose to prepare a suspension with a concentration of 2 mg/mL. Rats were orally administered leflunomide at a dose of 10 mg/kg via oral gavage. Blood samples (0.5 mL) were collected from the orbital venous plexus at 0.5, 1, 2, 4, 8, 12, 24, 48, and 72 hours post-administration, and placed in heparinized tubes. Plasma was separated by centrifugation at 3000 rpm for 10 minutes and stored at -80°C until analysis. Plasma concentrations of A771726 were determined using HPLC with a C18 column (250×4.6 mm, 5 μm). The mobile phase was a mixture of acetonitrile and 0.1% phosphoric acid (40:60, v/v) at a flow rate of 1 mL/min, and detection was performed at 280 nm [3] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Well absorbed, peak plasma concentrations appear 6-12 hours after dosing The active metabolite is eliminated by further metabolism and subsequent renal excretion as well as by direct biliary excretion. In a 28 day study of drug elimination (n=3) using a single dose of radiolabeled compound, approximately 43% of the total radioactivity was eliminated in the urine and 48% was eliminated in the feces. It is not known whether leflunomide is excreted in human milk. Many drugs are excreted in human milk, and there is a potential for serious adverse reactions in nursing infants from leflunomide. 0.13 L/kg Following oral administration of leflunomide, the drug is rapidly converted to A77 1726 in the GI mucosa and liver. Time to peak concentration: Approximately 6 to 12 hours. /M1 metabolite/ M1 metabolite is 80% bioavailable. Administration of leflunomide with a high-fat meal has no effect on the plasma concentration of M1. /M1 metabolite/ M1 has a low volume of distribution (Vss = 0.13 L/kg) and is extensively bound (>99.3%) to albumin in healthy subjects. Protein binding has been shown to be linear at therapeutic concentrations. The free fraction of M1 is slightly higher in patients with rheumatoid arthritis and approximately doubled in patients with chronic renal failure; the mechanism and significance of these increases are unknown. For more Absorption, Distribution and Excretion (Complete) data for LEFLUNOMIDE (8 total), please visit the HSDB record page. Metabolism / Metabolites Primarily hepatic. Leflunomide is converted to its active form following oral intake. Leflunomide is metabolized to M1 and other minor active metabolites. An active metabolite, 4-trifluoromethylaniline, is present in plasma at low concentrations. Although the specific site of leflunomide metabolism is unknown, it has been suggested that the gastrointestinal wall and liver play a role in the metabolism. The 3-unsubstituted isoxazole ring in the anti-inflammatory drug leflunomide undergoes a unique N-O bond cleavage to the active alpha-cyanoenol metabolite A771726, which resides in the same oxidation state as the parent. In vitro studies were conducted to characterize drug-metabolizing enzyme(s) responsible for ring opening and to gain insight into the mechanism of ring opening. ... Although A771726 formation in human liver microsomes or recombinant p4501A2 required NADPH, its formation was greatly reduced by oxygen or carbon monoxide, suggesting that the isoxazole ring opening was catalyzed by the p450Fe(II) form of the enzyme. A mechanism for the p450-mediated ring scission is proposed in which the isoxazole ring nitrogen or oxygen coordinates to the reduced form of the heme followed by charge transfer from p450Fe(II) to the C=N bond or deprotonation of the C3-H, which results in a cleavage of the N-O bond. Leflunomide has known human metabolites that include (E)-3-Hydroxy-2-methanimidoyl-N-[4-(trifluoromethyl)phenyl]but-2-enamide. Biological Half-Life 2 weeks 2 weeks /M1 metabolite/ 1. Leflunomide is rapidly metabolized to its active metabolite A771726 in vivo (in rats and humans). After oral administration of leflunomide to rats (10 mg/kg), the Tmax of A771726 is ~2 hours, and the elimination t1/2 is ~16 hours. In humans, the oral bioavailability of leflunomide is approximately 80%, and A771726 has a long elimination t1/2 of 14-18 days [3] ; 2. The plasma protein binding rate of A771726 (active metabolite of leflunomide) is >99% in both rats and humans, primarily binding to albumin [3] ; 3. Leflunomide is mainly metabolized in the liver by cytosolic enzymes (specific enzymes not identified in the literature), and the metabolites (including A771726) are primarily excreted in bile (approximately 70% of the dose) and partially excreted in urine (approximately 10-15% of the dose) [3] ; |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
Concurrent use with rifampin may increase the plasma concentration of leflunomide; caution is recommended. Concurrent use with these medications /hepatotoxic medications or methotrexate/ may increase the risk of side effects and medication-induced hepatic toxicity; in a small study evaluating the concurrent use of leflunomide (100 mg/day followed by 10 to 20 mg/day) and methotrexate (10 to 25 mg/week with folate), an increased risk of hepatotoxicity was reported; dosage adjustment may be needed. In vivo drug interaction studies have demonstrated a lack of a significant drug interaction between leflunomide and tri-phasic oral contraceptives, and cimetidine. M1 was shown to cause increases ranging from 13-50% in the free fraction of diclofenac, ibuprofen and tolbutamide at concentrations in the clinical range. In vitro studies of drug metabolism indicate that M1 inhibits CYP 450 2C9, which is responsible for the metabolism of many NSAIDs. M1 has been shown to inhibit the formation of 4'-hydroxydiclofenac from diclofenac in vitro. Concurrent use with these medications /activated charcoal, or cholestyramine/ will significantly decrease the plasma concentration of M1 by inhibiting gastrointestinal absorption. /M1 metabolite/ Non-Human Toxicity Values LD50 Rabbit oral 132 mg/kg LD50 Rat oral 235 mg/kg LD50 Mouse oral 445 mg/kg 1. In the CIA mouse model, oral administration of leflunomide at doses of 3 mg/kg/day and 10 mg/kg/day for 14 days did not cause obvious mortality or severe toxicity. However, a small number of mice in the high-dose group showed mild gastrointestinal symptoms (e.g., reduced food intake, loose stools) [3] ; 2. In a 4-week repeated-dose toxicity study in rats (oral administration of leflunomide at 10, 30, 50 mg/kg/day), the high-dose group (50 mg/kg/day) showed a significant increase in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels (indicative of liver injury), while no significant changes in renal function parameters (e.g., serum creatinine, blood urea nitrogen) were observed in any group [3] ; 3. Due to the high plasma protein binding rate of A771726, leflunomide may potentially interact with other highly protein-bound drugs (e.g., warfarin, phenytoin) by competing for binding sites [3] ; |
| 参考文献 |
[1]. Davis JP, et al. The immunosuppressive metabolite of leflunomide is a potent inhibitor of human dihydroorotate dehydrogenase. Biochemistry. 1996 Jan 30;35(4):1270-3.
[2]. Xu X, et al. Inhibition of protein tyrosine phosphorylation in T cells by a novel immunosuppressive agent, leflunomide. J Biol Chem. 1995 May 26;270(21):12398-403. [3]. Fox RI, et al. Mechanism of action for leflunomide in rheumatoid arthritis. Clin Immunol. 1999 Dec;93(3):198-208 |
| 其他信息 |
Therapeutic Uses
Antirheumatic Leflunomide is indicated to alleviate the signs and symptoms of rheumatoid arthritis and to slow joint impairment. /Included in US product labeling/ Leflunomide, a new oral immunomodulatory agent, is effective for the treatment of rheumatoid arthritis. Its mechanism of action in suppressing inflammation is based in its inhibition of dihydroorotate dehydrogenase, an enzyme responsible for de novo synthesis of pyrimidine containing ribonucleotides. It is the first disease-modifying antirheumatic drug approved for treatment of rheumatoid arthritis with an indication for retardation of joint damage by radiography. Side effects are generally mild and include diarrhea, rashes, reversible alopecia, and elevation of hepatic transaminases. Despite the concern about hepatotoxicity, combination use with methotrexate in treating patients with rheumatoid arthritis has been shown to be safe. Other autoimmune diseases in which leflunomide has been used successfully include Felty syndrome, vasculitis, Sjogren syndrome, Wegener granulomatosis, and bullous pemphigoid. Leflunomide has excellent antiviral activity against cytomegalovirus (CMV) in animal models and is considerably less expensive than intravenous ganciclovir. We used leflunomide in four consenting renal allograft recipients with symptomatic CMV disease, who were unable to afford ganciclovir and would otherwise remain untreated. This is the first report of efficacy of leflunomide in humans with CMV disease. They received loading dose of 100 mg of leflunomide once daily on days 1-3 and then 20 mg once daily for 3 months. All four patients were followed up three times weekly with physical examination, total leukocyte counts, blood urea and serum creatinine for a minimum period of 6 weeks. None of the patients showed drug related adverse events, alteration in cyclosporine levels, or decreased graft function, except one who developed leucopenia. Preliminary data presented suggests that leflunomide therapy for CMV disease is effective and could be used with careful monitoring in allograft recipients who cannot afford intravenous ganciclovir therapy. The duration of treatment and the role of leflunomide in secondary prophylaxis and in situations of ganciclovir resistance need to be studied further. Drug Warnings FDA Pregnancy Risk Category: X /CONTRAINDICATED IN PREGNANCY. Studies in animals or humans, or investigational or post-marketing reports, have demonstrated positive evidence of fetal abnormalities or risk which clearly outweights any possible benefit to the patient./ Because it can take up to 2 years for plasma concentrations of the active metabolite of leflunomide (A77 1726) to decrease to undetectable concentrations (less than 0.02 ug/mL) following discontinuance of leflunomide, the possibility that adverse effects or drug interactions associated with the drug could continue to occur even thought the patient is no longer receiving leflunomide should be considered. Opportunistic infections and serious infection, including sepsis and death, have been reported rarely in patients receiving leflunomide. Most serious infections reported in patients receiving leflunomide occurred in those receiving concomitant therapy with immunosuppressive agent and/or those with comorbid illness that, in addition to rheumatoid arthritis, could have predisposed them to infections. Leflunomide, a new immunomodulatory agent, was prescribed to a 67-year-old female patient with rheumatoid arthritis. Fifteen days later she developed diarrhea and elevated liver enzymes. A liver biopsy showed a pattern of acute hepatitis. The patient was homozygous for the rare CYP2C93 allele, which determines the slowest metabolic rate for CYP2C9 enzymatic activity, that is probably involved in the metabolism of leflunomide. Liver damage subsided in few weeks. This case illustrates the risk of hepatotoxicity by leflunomide and suggests that it is possibly related to CYP2C9 polymorphism. For more Drug Warnings (Complete) data for LEFLUNOMIDE (20 total), please visit the HSDB record page. Pharmacodynamics Leflunomide is a pyrimidine synthesis inhibitor indicated in adults for the treatment of active rheumatoid arthritis (RA). RA is an auto-immune disease characterized by high T-cell activity. T cells have two pathways to synthesize pyrimidines: the salvage pathways and the de novo synthesis. At rest, T lymphocytes meet their metabolic requirements by the salvage pathway. Activated lymphocytes need to expand their pyrimidine pool 7- to 8-fold, while the purine pool is expanded only 2- to 3-fold. To meet the need for more pyrimidines, activated T cells use the de novo pathway for pyrimidine synthesis. Therefore, activated T cells, which are dependent on de novo pyrimidine synthesis, will be more affected by leflunomide's inhibition of dihydroorotate dehydrogenase than other cell types that use the salvage pathway of pyrimidine synthesis. 1. Leflunomide is an immunosuppressive agent primarily used in the treatment of rheumatoid arthritis (RA). Its main mechanism of action is mediated by its active metabolite A771726, which inhibits DHODH—a key enzyme in the de novo pyrimidine biosynthesis pathway. Inhibition of DHODH reduces pyrimidine production, thereby inhibiting the proliferation of activated lymphocytes (T and B cells) that depend on de novo pyrimidine synthesis for rapid division [1, 3] ; 2. The inhibition of protein tyrosine phosphorylation in T cells by leflunomide (as observed in [2]) is an additional mechanism contributing to its immunosuppressive effect. Protein tyrosine phosphorylation is an early and critical step in T cell activation (e.g., following T cell receptor engagement), and inhibition of this process impairs T cell activation, proliferation, and cytokine secretion [2, 3] ; 3. In clinical settings, leflunomide has been shown to reduce joint inflammation, slow joint destruction, and improve physical function in patients with RA. However, regular monitoring of liver function is recommended due to the potential risk of liver injury at high doses [3] |
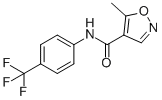
| 分子式 |
C12H9F3N2O2
|
|
|---|---|---|
| 分子量 |
270.21
|
|
| 精确质量 |
270.061
|
|
| 元素分析 |
C, 53.34; H, 3.36; F, 21.09; N, 10.37; O, 11.84
|
|
| CAS号 |
75706-12-6
|
|
| 相关CAS号 |
Leflunomide-d4;1189987-23-2
|
|
| PubChem CID |
3899
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 沸点 |
289.3±40.0 °C at 760 mmHg
|
|
| 熔点 |
163-168°C
|
|
| 闪点 |
128.8±27.3 °C
|
|
| 蒸汽压 |
0.0±0.6 mmHg at 25°C
|
|
| 折射率 |
1.541
|
|
| LogP |
1.95
|
|
| tPSA |
55.13
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
19
|
|
| 分子复杂度/Complexity |
327
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O=C(C1=C(C)ON=C1)NC2=CC=C(C(F)(F)F)C=C2
|
|
| InChi Key |
VHOGYURTWQBHIL-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C12H9F3N2O2/c1-7-10(6-16-19-7)11(18)17-9-4-2-8(3-5-9)12(13,14)15/h2-6H,1H3,(H,17,18)
|
|
| 化学名 |
5-methyl-N-[4-(trifluoromethyl)phenyl]-1,2-oxazole-4-carboxamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (9.25 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液; 超声和加热处理
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (9.25 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (9.25 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7008 mL | 18.5041 mL | 37.0083 mL | |
| 5 mM | 0.7402 mL | 3.7008 mL | 7.4017 mL | |
| 10 mM | 0.3701 mL | 1.8504 mL | 3.7008 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT06228443 | Not yet recruiting | Drug: Leflunomide 20 mg Film-coated Tablet |
Healthy Volunteer | International Bio service | April 1, 2024 | Phase 1 |
| NCT04361214 | Terminated | Drug: Leflunomide | COVID-19 | University of Chicago | May 5, 2020 | Phase 1 |
| NCT05937191 | Recruiting | Drug: Leflunomide Drug: Steroid Drug |
Idiopathic Pulmonary Hemosiderosis Leflunomide |
Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University |
June 1, 2023 | Phase 1 Phase 2 |
| NCT03709446 | Recruiting | Drug: Leflunomide | Breast Neoplasms Breast Diseases |
Joseph Sparano | April 16, 2019 | Phase 1 Phase 2 |
|
|---|
|
|