| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
| 靶点 |
Lesinurad (RDEA594) is a selective urate reabsorption inhibitor that primarily targets urate transporter 1 (URAT1, SLC22A12) with an IC50 of 1.7 μM for inhibiting urate uptake in URAT1-expressing cells; it also inhibits organic anion transporter 1 (OAT1, SLC22A6, IC50 = 6.4 μM) and OAT3 (SLC22A8, IC50 = 2.8 μM), and shows weak inhibition of OATP1B1 (SLCO1B1, IC50 > 100 μM), OATP1B3 (SLCO1B3, IC50 > 100 μM), MRP2 (ABCC2, IC50 > 100 μM), and BCRP (ABCG2, IC50 = 45 μM) [1]
Lesinurad targets URAT1 as the primary molecular target for urate-lowering activity [2] Lesinurad (RDEA594) is a potent inhibitor of URAT1-mediated urate reabsorption (IC50 not specified in the abstract), and its prodrug RDEA806 is metabolized to RDEA594 in vivo [3] |
|---|---|
| 体外研究 (In Vitro) |
Lesinurad是一种全新的选择性尿酸重吸收(SURI)试剂。发现肾转运蛋白 OAT1 和 OAT3 利用 lesinurad 作为底物;发现它们的 Km 值分别为 0.85 和 2 μM [1]。一种可提高近端肾小管尿酸盐排泄的 URAT1 和 OAT 染料称为 lesinurad (RDEA594) [2]。 Lesinurad (RDEA594) 是一种潜在有效的降尿酸药物,可抑制 CYP2C9 和 CYP2C8,IC50 值分别为 14.4 μM 和 16.2 μM,并通过防止尿酸再浓缩而显示出强大的 p450 特性。 Lesinurad 对 CYP1A2、CYP2C19 和 CYP2D6 的 IC50 为 100 μM[3]。
雷西纳德以剂量依赖方式抑制稳定表达人URAT1的HEK293细胞对[¹⁴C]尿酸的摄取,IC50为1.7 μM;同时抑制表达OAT1的细胞对[³H]对氨基马尿酸(PAH)的摄取(IC50=6.4 μM),以及表达OAT3的细胞对[³H]雌酮-3-硫酸盐(E3S)的摄取(IC50=2.8 μM)。浓度高达100 μM时,对OATP1B1或OATP1B3介导的[³H]普伐他汀摄取无显著抑制作用,仅微弱抑制BCRP介导的[³H]米托蒽醌外排(IC50=45 μM)和MRP2介导的[³H]甲氨蝶呤外排(IC50>100 μM)。在人肾近端小管细胞(HRPTC)中,10 μM的雷西纳德可使尿酸重吸收减少约40%,且不影响其他有机阴离子(如PAH、E3S)的摄取 [1] 雷西纳德作为选择性URAT1抑制剂阻断肾脏尿酸重吸收,进而增加尿酸排泄;在人肾组织制备物的体外实验中,其对URAT1的选择性高于其他尿酸转运体(如GLUT9),对肾脏电解质转运体无显著脱靶效应 [2] 雷西纳德(RDEA594)的体外降尿酸活性优于其前药RDEA806:RDEA594在纳摩尔至低微摩尔浓度下即可抑制URAT1介导的尿酸摄取,而RDEA806对URAT1无直接抑制活性,需代谢激活为RDEA594后才具有生物活性 [3] |
| 体内研究 (In Vivo) |
与其前药 RDEA806 相比,lesinurad (RDEA594) 表现出更优异的药代动力学。单剂量 300-800 mg RDEA806 的药理作用与 100 mg 剂量 Lesinurad 所表现出的药理作用相当[3]。
大鼠口服雷西纳德(10、30、100 mg/kg)可剂量依赖性增加尿尿酸排泄,分别提升25%、60%和110%,给药后4小时血清尿酸水平分别降低15%、35%和55%。在氧酸钾诱导的大鼠高尿酸血症模型中,口服30 mg/kg雷西纳德可在2小时内使血清尿酸水平降低40%,且该效应持续8小时。雷西纳德(30 mg/kg)与丙磺舒(50 mg/kg)联用,可协同增加尿尿酸排泄(较雷西纳德单药的60%提升至180%) [1] 在II/III期临床试验中,雷西纳德(200 mg或400 mg每日一次)联合黄嘌呤氧化酶抑制剂(XOIs,如别嘌醇、非布司他),可显著降低单用XOIs未达目标血尿酸的痛风高尿酸血症患者的血尿酸(sUA)水平:200 mg剂量可额外降低sUA 1.8 mg/dL,400 mg剂量在12周时可额外降低sUA 2.3 mg/dL。III期试验(CLEAR 1)显示,雷西纳德200 mg+别嘌醇组有56%的患者sUA<6 mg/dL,而别嘌醇单药组仅27%(P<0.001) [2] 比格犬口服雷西纳德(RDEA594,5 mg/kg)后,血药峰浓度(Cmax)为2.1 μg/mL,药时曲线下面积(AUC0-24h)为8.6 μg·h/mL,终末半衰期(t1/2)为3.2小时;而其前药RDEA806(5 mg/kg)的Cmax为1.5 μg/mL,AUC0-24h为5.2 μg·h/mL,t1/2为1.8小时,且仅30%转化为RDEA594。小鼠口服雷西纳德(10 mg/kg)后3小时血清尿酸水平降低45%,而RDEA806(10 mg/kg)因代谢不完全仅使sUA降低15% [3] |
| 酶活实验 |
1. URAT1抑制实验:将表达人URAT1的HEK293细胞膜制备物与[¹⁴C]尿酸(0.1 μM)及系列浓度的雷西纳德(0.1-100 μM)在转运缓冲液中37℃孵育10分钟;在1 mM丙磺舒存在下测定非特异性摄取,通过液体闪烁计数检测放射性,利用剂量-反应曲线计算尿酸摄取抑制的IC50(N=3次独立实验,每次设3个复孔) [1]
2. OAT1/OAT3抑制实验:将表达OAT1或OAT3的HEK293细胞膜与[³H]PAH(OAT1底物,0.5 μM)或[³H]E3S(OAT3底物,0.1 μM)及雷西纳德(0.1-100 μM)按URAT1实验方法孵育,通过非线性回归分析确定OAT1和OAT3抑制的IC50值 [1] 3. BCRP/MRP2外排实验:将负载[³H]米托蒽醌(BCRP底物)或[³H]甲氨蝶呤(MRP2底物)的BCRP/MRP2表达细胞内翻膜囊泡,与雷西纳德(0.1-100 μM)37℃孵育20分钟,检测外排的放射性并计算转运体抑制的IC50 [1] |
| 细胞实验 |
1. 表达URAT1的HEK293细胞摄取实验:将稳定转染人URAT1的HEK293细胞接种于24孔板,血清饥饿2小时后,与[¹⁴C]尿酸(0.1 μM)及系列浓度的雷西纳德(0.1-100 μM)在HBSS缓冲液中37℃孵育10分钟;用冰浴HBSS终止反应,通过液体闪烁计数检测细胞内放射性,按蛋白含量归一化摄取量并计算相对对照组的抑制百分比 [1]
2. 人肾近端小管细胞(HRPTC)实验:将原代HRPTC接种于胶原包被板,用雷西纳德(1、10、50 μM)处理1小时后加入[¹⁴C]尿酸(0.5 μM),15分钟后检测尿酸摄取;同时评估其他有机阴离子(PAH、E3S)的摄取,以分析雷西纳德的选择性 [1] 3. 转运体介导的药物相互作用实验:将表达OATP1B1/OATP1B3的HEK293细胞与雷西纳德(0.1-100 μM)及[³H]普伐他汀(0.5 μM)孵育30分钟,检测普伐他汀摄取量,判断雷西纳德是否干扰肝脏摄取转运体 [1] |
| 动物实验 |
1. Rat hyperuricemia model: Male Sprague-Dawley rats (200-250 g) were randomly divided into vehicle and Lesinurad treatment groups (10, 30, 100 mg/kg). Hyperuricemia was induced by intraperitoneal injection of potassium oxonate (250 mg/kg) 1 hour before Lesinurad administration. Lesinurad was formulated in 0.5% methylcellulose and administered by oral gavage. Blood samples were collected from the tail vein at 0, 2, 4, 6, and 8 hours post-dosing to measure serum urate levels using a uric acid assay kit. Urine was collected over 8 hours to quantify urinary urate excretion [1]
2. Rat drug interaction model: Rats were co-administered Lesinurad (30 mg/kg) and probenecid (50 mg/kg) by oral gavage. Urine and blood samples were collected at 4 hours post-dosing to measure urate levels and evaluate synergistic effects on urate excretion [1] 3. Beagle dog pharmacokinetic study: Male beagle dogs (8-10 kg) were given a single oral dose of Lesinurad (RDEA594, 5 mg/kg) or RDEA806 (5 mg/kg) formulated in 10% ethanol/PEG400. Blood samples were collected from the cephalic vein at 0, 0.5, 1, 2, 4, 6, 8, 12, and 24 hours post-dosing. Plasma concentrations of RDEA594 and RDEA806 were measured by LC-MS/MS, and pharmacokinetic parameters (Cmax, AUC, t1/2) were calculated using non-compartmental analysis [3] 4. Mouse urate-lowering efficacy study: Male C57BL/6 mice (20-25 g) were administered Lesinurad (10 mg/kg) or RDEA806 (10 mg/kg) by oral gavage. Serum urate levels were measured at 1, 3, 6, and 12 hours post-dosing using a colorimetric assay [3] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Oral lesinurad is rapidly absorbed, reaching maximum plasma concentrations (Cmax) within 1–4 h following the administration a single 200 mg dose (in either the fed or fasted state). Within 7 days following single dosing of radiolabeled lesinurad, 63% of administered radioactive dose was recovered in urine and 32% of administered radioactive dose was recovered in feces. Most of the radioactivity recovered in urine (> 60% of dose) occurred in the first 24 hours. Unchanged lesinurad in urine accounted for approximately 30% of the dose. The mean steady state volume of distribution of lesinurad was approximately 20 L following intravenous dosing. Metabolism / Metabolites Lesinurad undergoes oxidative metabolism mainly via the polymorphic cytochrome P450 CYP2C9 enzyme. Lesinurad is rapidly absorbed after oral administration in rats, with a Cmax reached at 1 hour and an oral bioavailability of approximately 85%. It is widely distributed in tissues, with the highest concentrations in the kidney, liver, and small intestine, and low concentrations in the brain (brain-to-plasma ratio < 0.1). Lesinurad is primarily metabolized by CYP3A4 and UGT2B7 in the liver, with approximately 70% of the dose excreted in urine (15% as unchanged drug) and 25% in feces within 48 hours. Its terminal half-life in rats is 2.5 hours, and in humans, the t1/2 is approximately 5-8 hours after a single 200 mg oral dose [1] In humans, Lesinurad has an oral bioavailability of ~90% after a 200 mg dose, with a Cmax of 1.2 μg/mL and AUC0-24h of 6.8 μg·h/mL. It is highly bound to human plasma proteins (99%), primarily to albumin. The major metabolic pathways are oxidation (CYP3A4) and glucuronidation (UGT2B7), with no active metabolites other than the parent compound. Approximately 60% of the dose is excreted in urine within 72 hours, with 20% as unchanged drug [2] Lesinurad (RDEA594) has superior pharmacokinetic properties compared to its prodrug RDEA806 in dogs: RDEA594 has a 40% higher Cmax, 65% higher AUC, and 78% longer t1/2 than RDEA806. RDEA806 is rapidly but incompletely metabolized to RDEA594 in vivo, with a bioactivation efficiency of only 30% in dogs and 25% in humans [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In large clinical trials, serum enzyme elevations were rare during lesinurad therapy and no more common than with placebo, and no instances of clinically apparent liver injury attributable to lesinurad were reported. Clinical experience with lesinurad therapy has been limited, but there have yet to be reports of clinically apparent liver injury attributable to its use. Likelihood score: E (unlikely cause of clinically apparent liver injury). Protein Binding Lesinurad is extensively bound to proteins in plasma (greater than 98%), mainly to albumin. In in vitro cytotoxicity assays, Lesinurad showed no significant toxicity to HRPTC or HEK293 cells at concentrations up to 500 μM (CC50 > 500 μM). In a 28-day subchronic toxicity study in rats, oral administration of Lesinurad (50, 100, 200 mg/kg/day) caused no significant changes in body weight, food intake, or hematological parameters. Renal histology showed mild tubular vacuolization at the 200 mg/kg dose, which was reversible after a 14-day recovery period. No hepatotoxicity was observed, as serum ALT/AST levels remained normal [1] In clinical trials, Lesinurad was well-tolerated at doses of 200 mg/day; the most common adverse events (AEs) were headache (12%), fatigue (8%), and urinary tract infection (6%). At the 400 mg/day dose, the incidence of renal-related AEs (e.g., increased serum creatinine, acute kidney injury) increased to 9% (vs 3% with 200 mg/day). Lesinurad has a low potential for drug-drug interactions: it does not inhibit or induce CYP450 enzymes (CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A4) in vitro, and co-administration with allopurinol/febuxostat did not alter their plasma concentrations [2] In acute toxicity studies, the median lethal dose (LD50) of Lesinurad in mice was >2000 mg/kg after oral administration, and >500 mg/kg after intraperitoneal administration. RDEA806 had a similar LD50 profile, with no increased toxicity compared to RDEA594. In a 14-day toxicity study in dogs, Lesinurad (100 mg/kg/day) caused mild increases in serum urea and creatinine, which resolved after drug withdrawal [3] |
| 参考文献 |
|
| 其他信息 |
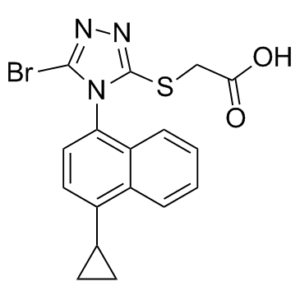
Lesinurad is a member of the class of triazoles that is [(3-bromo-1,2,4-triazol-5-yl)sulfanyl]acetic acid substituted at position 1 of the triazole ring by a 4-cyclopropylnaphthalen-1-yl group. Used for treatment of gout. It has a role as a uricosuric drug. It is a member of triazoles, a member of naphthalenes, a member of cyclopropanes, an organobromine compound, an aryl sulfide and a monocarboxylic acid.
Lesinurad is an oral uric acid transporter 1 (URAT1) inhibitor indicated for the treatment of hyperuricemia associated with gout. It reduces serum uric acid concentration through the inhibition of URAT1, an enzyme responsible for reuptake of uric acid from the renal tubule, and OAT4, another uric acid transporter associated with diuretic-induced hyperuricemia. Marketed as the product Zurampic, it is indicated for use in combination with a xanthine oxidase inhibitor for the treatment of hyperuricemia associated with gout in patients who have not achieved target serum uric acid levels with a xanthine oxidase inhibitor alone. In August 2017, a combination oral therapy consisting of lesinurad and [DB00437] was FDA-approved under the brand name Duzallo indicated for the treatment of gout-related hyperuricemia in patients with uncontrolled gout. Lesinurad is an Urate Transporter 1 Inhibitor. The mechanism of action of lesinurad is as an Urate Transporter 1 Inhibitor, and Cytochrome P450 3A Inducer. Lesinurad is a selective inhibitor of uric acid reabsorption which is used in combination with other agents in the therapy of gout. Lesinurad has had limited clinical use, but has not been associated with serum enzyme elevations during therapy or with instances of clinically apparent liver injury. See also: Allopurinol; Lesinurad (component of). Drug Indication For use, in combination with a xanthine oxidase inhibitor, for the treatment of hyperuricemia associated with gout in patients who have not achieved target serum uric acid levels with a xanthine oxidase inhibitor alone. FDA Label Zurampic, in combination with a xanthine oxidase inhibitor, is indicated in adults for the adjunctive treatment of hyperuricaemia in gout patients (with or without tophi) who have not achieved target serum uric acid levels with an adequate dose of a xanthine oxidase inhibitor alone. , Prevention of hyperuricaemia, Treatment of hyperuricaemia Mechanism of Action Lesinurad inhibits the activity of uric acid transporter 1 (URAT1) and organic anion transporter 4 (OAT4). URAT1 is a major transporter enzyme responsible for reuptake of uric acid from the renal tubules; inhibition of URAT1 function thereby increases excretion of uric acid. Pharmacodynamics Dose-dependent reductions in serum uric acid levels and increases in urinary uric acid excretion have been observed following single and multiple oral doses of lesinurad. Lesinurad is a first-in-class selective urate reabsorption inhibitor (SURI) developed for the treatment of hyperuricemia in patients with gout. Its mechanism of action involves blocking URAT1-mediated urate reabsorption in the proximal renal tubule, thereby increasing urate excretion and reducing serum urate levels. It is approved for use in combination with XOIs in patients who fail to achieve target sUA with XOIs alone [1] Lesinurad is indicated for the treatment of hyperuricemia associated with gout in adults, in combination with an XOI (allopurinol or febuxostat). It is not recommended as monotherapy due to limited efficacy and increased renal risk at higher doses. The recommended clinical dose is 200 mg once daily, taken with food to reduce gastrointestinal irritation [2] Lesinurad (RDEA594) is the active metabolite of RDEA806, a prodrug designed to improve oral bioavailability. RDEA806 is rapidly hydrolyzed to RDEA594 in the liver by carboxylesterases, but its bioactivation is incomplete, leading to the development of RDEA594 as the clinical candidate. Lesinurad has been evaluated in multiple phase III trials (CLEAR 1, CLEAR 2, CRYSTAL) and was approved by the FDA in 2015 [3] |
| 分子式 |
C17H14BRN3O2S
|
|
|---|---|---|
| 分子量 |
404.28
|
|
| 精确质量 |
402.998
|
|
| CAS号 |
878672-00-5
|
|
| 相关CAS号 |
Lesinurad sodium;1151516-14-1
|
|
| PubChem CID |
53465279
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.72±0.1 g/cm3
|
|
| 沸点 |
643.7±65.0 °C at 760 mmHg
|
|
| 闪点 |
343.1±34.3 °C
|
|
| 蒸汽压 |
0.0±2.0 mmHg at 25°C
|
|
| 折射率 |
1.776
|
|
| LogP |
5.96
|
|
| tPSA |
93.31
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
24
|
|
| 分子复杂度/Complexity |
479
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
FGQFOYHRJSUHMR-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C17H14BrN3O2S/c18-16-19-20-17(24-9-15(22)23)21(16)14-8-7-11(10-5-6-10)12-3-1-2-4-13(12)14/h1-4,7-8,10H,5-6,9H2,(H,22,23)
|
|
| 化学名 |
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.18 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.18 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.18 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4735 mL | 12.3677 mL | 24.7353 mL | |
| 5 mM | 0.4947 mL | 2.4735 mL | 4.9471 mL | |
| 10 mM | 0.2474 mL | 1.2368 mL | 2.4735 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Lesinurad and Allopurinol Combination Extension Study in Gout
CTID: NCT01808131
Phase: Phase 3 Status: Completed
Date: 2017-01-24
Median plasma concentration profiles for total atorvastatin (including metabolites) following a single oral dose of atorvastatin 40mg in the absence or presence of a single dose of lesinurad 200mg (a) or 400mg (b), and plasma concentration profile of metformin following a single dose of metformin 850mg (c) or plasma concentration profile of furosemide following a single dose of furosemide 40mg (d) in the absence or presence of a single dose of lesinurad 400mg.Clin Drug Investig.2016 Jun;36(6):443-52. |
|---|
 Uric acid pathway and action site of urate-lowering therapies. *Drugs in italics are agents still under development or still not approved. **Dashed arrow representing lack of metabolic step in humans, due to evolutionary loss of uricase enzyme. |
 enal anion transporters involved in urate reabsorption in the proximal tubule and action sites of existing and novel uricosuric agents. *Drugs in italics are agents still under development or still not approved. |