| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg | |||
| Other Sizes |
| 靶点 |
Antiviral; Arenavirus envelope glycoprotein
|
|---|---|
| 体外研究 (In Vitro) |
LHF-535是一种小分子病毒进入抑制剂,针对沙粒病毒包膜糖蛋白(GP)。LHF-535对多种出血热沙粒病毒表现出强大的抗病毒活性。 LHF-535 抑制 Lassa GP 假型慢病毒,IC50 为 0.1-0.3 nM[2]。
|
| 体内研究 (In Vivo) |
LHF-535(3、10 或 30 mg/kg;口服;每日;14 天)显着降低血浆、脾脏和肝脏中的病毒滴度,同时保护小鼠免受致命的塔卡里布病毒攻击。延迟 LHF-535 的第一剂(感染后 1、2 或 3 天注射 LHF-535(10 mg/kg)也会导致存活率增加,这表明 LHF-535 作为小鼠暴露后治疗剂的有效性[2]。
|
| 酶活实验 |
抗病毒检测[2]
Junín病毒产量减少测定法[2] 进行病毒产量降低(VYR)实验,以确定Junín-Romero野生型和疫苗株对LHF-535的敏感性。在感染前,将不同浓度的LHF-535添加到含有70-80%融合Vero细胞的测试孔中,感染复数(MOI)约为0.002。培养板培养3天,此时将感染病毒的培养板冷冻和解冻,并收集培养上清液用于感染病毒的终点滴定。将样品接种在Vero细胞上,并在感染后第10天测量视觉细胞病变效应。LHF-535针对Candid#1和Romero菌株进行一式三份的测试。接种疫苗的人员在BSL-3+实验室对Junín病毒的致病性Romero株进行了研究。 Tacaribe病毒抗病毒试验[2] 接种在96孔板中的Vero细胞(每孔5000个细胞)在DMSO中加入一式三份的系列化合物稀释液后,以0.1的MOI感染Tacaribe病毒。3天后,从细胞裂解物(Promega SV 96总RNA分离系统)中提取RNA,通过qRT-PCR和比较CT方法评估Tacaribe病毒的RNA[40]。简言之,提取的RNA用于用高容量RNA-to-cDNA试剂盒(Thermo Fisher Scientific)产生cDNA。对于基于TaqMan的qPCR,使用TaqMan-Fast Advanced Master Mix(Thermo Fisher Scientific)以及引物和双重标记的TaqMan-探针组(靶向GP的约100个核苷酸区域(nt 809–912))对cDNA进行反应。使用赛默飞世尔科学公司的18S rRNA(VIC/MGB探针)作为内部控制。 假型病毒抑制[2] 将293T细胞接种在不透明的384孔板中(每孔4000个细胞)。第二天,通过HP D300e数字分配器将LHF-535(溶解在DMSO中)和单独的DMSO分配到所有孔中的最终浓度为0.2%的DMSO。随后加入固定体积的慢病毒假病毒粒子,在37°C下孵育3天,并测量萤光素酶活性(Promega Bright Glo萤光素酶测定系统)。试验浓度一式四份。对每种浓度或对照(阳性对照单独接受DMSO,阴性对照模拟感染)的发光进行平均,并使用XLfit计算50%的有效浓度。多次重复实验以建立平均值(几何平均值)EC50;重复实验,直到多个实验的平均值的标准误差小于平均值的四分之一。 |
| 细胞实验 |
细胞和病毒
Vero和293T细胞从美国典型培养物保藏中心(ATCC;Manassas,VA)获得。Vero细胞维持在补充有10%胎牛血清的最低必需培养基(MEM)中(HyClone Thermo Scientific,Logan,UT)。293T细胞维持在补充有10%胎牛血清、2mM L-谷氨酰胺、青霉素(100U/ml)和链霉素(100μg/ml)的Dulbecco改良Eagle培养基(DMEM)中。Tacaribe病毒株TRVL 11573从ATCC获得。Junín病毒的Candid#1疫苗株由Robert Tesh(德克萨斯州加尔维斯顿得克萨斯大学医学分院新发病毒和虫媒病毒世界参考中心)提供。Candid#1病毒原液(~108PFU/ml)在非洲绿猴肾细胞(来自ATCC的BS-C-1)中传代一次,在Vero细胞中传代两次后,由澄清的裂解物产生。Junín病毒的Romero株的分子克隆由Slobodan Paessler(德克萨斯大学医学分院,德克萨斯州加尔维斯顿)提供。病毒在幼仓鼠肾成纤维细胞(从ATCC获得的BHK-21)中被拯救,并且从Vero细胞中的单个传代制备储备物(~108PFU/ml)。接种疫苗的人员在BSL-3+实验室对Junín病毒的致病性Romero株进行了研究。[2] |
| 动物实验 |
Animal Model: IFN-α/β and-γ receptor-deficient AG129 mice[2]
Dosage: 3, 10, or 30 mg/kg/day Administration: Orally; daily; 14 days Result: Effective as a post-exposure therapeutic. Tacaribe virus in vivo model[2] AG129 mice are IFN-α/β and–γ receptor-deficient mice. They were a kind gift from Michael Diamond (Washington University in St. Louis). For Tacaribe virus studies, we used mice that were sex- and age-matched (6–8 weeks old). All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) and were conducted at Kineta in a BSL-2 facility. Experimental groups were sized (as specified in the figure legends) to allow for statistical analysis, and all animals were included in the analysis. All animal experiments were conducted in a non-blinded fashion. For the LHF-535 dose titration study, mice were sorted into survival and titer arms and challenged by intraperitoneal (i.p.) injection with 200 PFU of Tacaribe virus. In the survival arm, mice were dosed orally with LHF-535 at 3, 10, or 30 mg/kg/day or with vehicle alone for 14 days with the first dose 30 min prior to infection. Micronized LHF-535 was suspended in 0.5% Methocel E15 and 1% Tween 80. The mice were observed for signs of morbidity and mortality. For the titer arm, mice were sacrificed at 7 days post-challenge; plasma, liver, and spleen samples were collected for assaying virus titers. For the delayed treatment studies, AG129 mice were split into five groups with each receiving LHF-535 30 min prior, and 24, 48, and 72 h post infection along with a vehicle control group. All mice were challenged by i.p. injection with 200 PFU of Tacaribe virus and dosing ceased 14 days post-challenge. The mice were observed for signs of morbidity and mortality and were humanely removed from study if there were clinical observations of inactivity, labored breathing, or excessive weight loss (≥20%). For the pathogenesis studies (LD50 determination), AG129 mice were infected with wild-type or mutant Tacaribe virus via i.p. injection using 10-fold serial dilutions of virus. The Reed-Muench method was used for LD50 calculations |
| 参考文献 | |
| 其他信息 |
Arenaviruses are a significant cause of hemorrhagic fever, an often-fatal disease for which there is no approved antiviral therapy. Lassa fever in particular generates high morbidity and mortality in West Africa, where the disease is endemic, and a recent outbreak in Nigeria was larger and more geographically diverse than usual. We are developing LHF-535, a small-molecule viral entry inhibitor that targets the arenavirus envelope glycoprotein, as a therapeutic candidate for Lassa fever and other hemorrhagic fevers of arenavirus origin. Using a lentiviral pseudotype infectivity assay, we determined that LHF-535 had sub-nanomolar potency against the viral envelope glycoproteins from all Lassa virus lineages, with the exception of the glycoprotein from the LP strain from lineage I, which was 100-fold less sensitive than that of other strains. This reduced sensitivity was mediated by a unique amino acid substitution, V434I, in the transmembrane domain of the envelope glycoprotein GP2 subunit. This position corresponds to the attenuation determinant of Candid#1, a live-attenuated Junín virus vaccine strain used to prevent Argentine hemorrhagic fever. Using a virus-yield reduction assay, we determined that LHF-535 potently inhibited Junín virus, but not Candid#1, and the Candid#1 attenuation determinant, F427I, regulated this difference in sensitivity. We also demonstrated that a daily oral dose of LHF-535 at 10 mg/kg protected mice from a lethal dose of Tacaribe virus. Serial passage of Tacaribe virus in LHF-535-treated Vero cells yielded viruses that were resistant to LHF-535, and the majority of drug-resistant viruses exhibited attenuated pathogenesis. These findings provide a framework for the clinical development of LHF-535 as a broad-spectrum inhibitor of arenavirus entry and provide an important context for monitoring the emergence of drug-resistant viruses.[2]
|
| 分子式 |
C27H28N2O2
|
|---|---|
| 分子量 |
412.523427009583
|
| 精确质量 |
412.22
|
| 元素分析 |
C, 78.61; H, 6.84; N, 6.79; O, 7.76
|
| CAS号 |
1450929-77-7
|
| 相关CAS号 |
(E)-LHF-535
|
| PubChem CID |
71711529
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| LogP |
5.8
|
| tPSA |
47.3Ų
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
31
|
| 分子复杂度/Complexity |
588
|
| 定义原子立体中心数目 |
0
|
| SMILES |
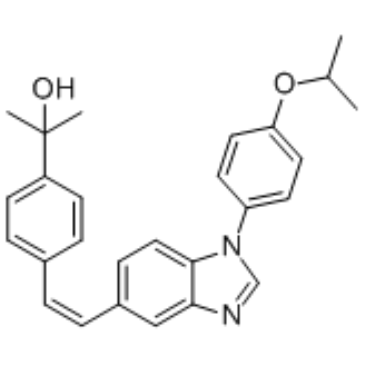
CC(C)OC1=CC=C(C=C1)N2C=NC3=C2C=CC(=C3)/C=C\C4=CC=C(C=C4)C(C)(C)O
|
| InChi Key |
DBNZTRPIBJSUIX-WAYWQWQTSA-N
|
| InChi Code |
InChI=1S/C27H28N2O2/c1-19(2)31-24-14-12-23(13-15-24)29-18-28-25-17-21(9-16-26(25)29)6-5-20-7-10-22(11-8-20)27(3,4)30/h5-19,30H,1-4H3/b6-5-
|
| 化学名 |
(Z)-2-(4-(2-(1-(4-Isopropoxyphenyl)-1H-benzo[d]imidazol-5-yl)vinyl)phenyl)propan-2-ol
|
| 别名 |
LHF535; LHF 535; LHF-535
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~150 mg/mL (~363.62 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.42 mg/mL (5.87 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 24.2 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.42 mg/mL (5.87 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 24.2 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4241 mL | 12.1206 mL | 24.2412 mL | |
| 5 mM | 0.4848 mL | 2.4241 mL | 4.8482 mL | |
| 10 mM | 0.2424 mL | 1.2121 mL | 2.4241 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|
|
|