| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
GLP-1 receptor
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:Lixisenatide 保护 Ins-1 细胞(大鼠来源的 β 细胞系)免受脂质和细胞因子诱导的细胞凋亡。更重要的是,Lixisenatide 还可以防止脂毒性诱导的人胰岛胰岛素耗竭,并在体外保护胰岛素的产生、储存和胰腺 β 细胞功能。在过表达人 GLP-1 受体的 CHO-K1 细胞中进行的结合研究表明,Lixisenatide 是一种非常有效的选择性 GLP-1 受体激动剂——Lixisenatide 的结合亲和力 (Ki = 1.33 ± 0.22 nM) 约为 4 倍人类 GLP-1 的值 (Ki = 5.09 ± 1.19 nM)。在 80 多种不同的结合测定中,利西拉来未表现出与其他潜在药物靶标的任何相关相互作用,证实了其对 GLP-1 受体的高选择性激酶测定:利西拉来是胰高血糖素样肽-1 受体的短效激动剂(GLP-1R) 在体外受体结合研究中对人 GLP-1 受体的 IC50 值为 1.4 nM。它可以通过每日一次的方案进行给药。细胞测定:Lixisenatide 能够保护 Ins-1 细胞(大鼠来源的 β 细胞系)免受脂质和细胞因子诱导的细胞凋亡。更重要的是,Lixisenatide 还可以防止脂毒性诱导的人胰岛胰岛素耗竭,并在体外保护胰岛素的产生、储存和胰腺 β 细胞功能。
|
| 体内研究 (In Vivo) |
利西拉来的半衰期为2-4小时,与长效GLP-1肽、利拉鲁肽和阿必鲁肽相比,它被归类为短效GLP-1受体激动剂。利西拉来可以显着改善葡萄糖刺激的胰岛素分泌。在健康血糖正常的狗中,单次皮下注射利司那肽在口服葡萄糖激发后会产生剂量依赖性的血浆葡萄糖降低,与安慰剂相比,在不增加胰岛素浓度的情况下,餐后血糖波动显着降低 67%。利西拉来对狗餐后血糖波动的影响至少部分与抑制胃排空和延迟肠道葡萄糖吸收有关。在 db/db 小鼠和 ZDF 大鼠中也证实了口服葡萄糖激发后血浆葡萄糖的剂量依赖性降低。重要的是,这种活性是葡萄糖依赖性的,在生理葡萄糖浓度下没有影响。在 db/db 小鼠中,长期服用利司那肽可防止对照动物中观察到的糖耐量进行性恶化,并与糖化血红蛋白 (HbA1c) 的剂量依赖性显着降低相关。在 ZDF 大鼠中,与对照动物相比,连续皮下注射 lixisenatide 50 μg/kg/天 12 周可显着降低基础血糖并改善口服葡萄糖耐量。它没有降血糖作用,并且不会改变血糖正常大鼠的 HbA1c。利西拉来可以通过刺激胰岛细胞增殖和新生以及抑制胰岛细胞凋亡来维持β细胞质量和功能。
|
| 酶活实验 |
体外受体结合研究表明,Lixisenatide 是胰高血糖素样肽-1 受体 (GLP-1R) 的短效激动剂,对人 GLP-1 受体的 IC50 值为 1.4 nM。可以采用每日一种的方案来给药。
GLP-1受体结合研究。[2] 简而言之,收获了携带人重组GLP-1受体的CHO-K1细胞。含有受体的膜部分被纯化并用于。。。 ZP10A与人GLP-1受体的结合。GLP-1(7-36)酰胺与CHO-K1细胞中表达的人GLP-1受体结合的半数最大抑制(IC50)浓度为5.5±1.3 nM,该值在GLP-1与胰岛细胞系中发现的内源性受体和COS-7细胞中表示的重组受体结合的报告范围内(Goke和Conlon,1988;Goke等人,1989;Fehmann和Habener,1991;Thorens,1992;Wheeler等人,1993)。IC50。。。 |
| 细胞实验 |
Lixisenatide 能够预防 Ins-1 细胞(一种源自大鼠的 β 细胞系)中由脂质和细胞因子引起的细胞凋亡。更重要的是,利西拉来可以在体外维持胰岛素合成、储存和胰腺β细胞功能,并防止脂毒性诱导的人胰岛胰岛素耗竭。
|
| 动物实验 |
Pphosphate-buffered saline, pH 7.4; 0.01, 0.1, 1, 10, and 100 nmol/kg; i.p.
Male db/db mice C57BLKS/J-Leprdb/Leprdb ZP10A demonstrated dose-dependent improvement of glucose tolerance with an ED50 value of 0.02 nmol/kg i.p. in an oral glucose tolerance test (OGTT) in diabetic db/db mice. After 42 days of treatment, ZP10A dose-dependently (0, 1, 10, or 100 nmol/kg b.i.d.; n = 10/group), decreased glycosylated hemoglobin (HbA1C) from 8.4 +/- 0.4% (vehicle) to a minimum of 6.2 +/- 0.3% (100 nmol/kg b.i.d.; p < 0.05 versus vehicle) in db/db mice. Fasting blood glucose (FBG), glucose tolerance after an OGTT, and HbA1C levels were significantly improved in mice treated with ZP10A for 90 days compared with vehicle-treated controls. Interestingly, these effects were preserved 40 days after drug cessation in db/db mice treated with ZP10A only during the first 50 days of the study. Real-time polymerase chain reaction measurements demonstrated that the antidiabetic effect of early therapy with ZP10A was associated with an increased pancreatic insulin mRNA expression relative to vehicle-treated mice. In conclusion, long-term treatment of diabetic db/db mice with ZP10A resulted in a dose-dependent improvement of FBG, glucose tolerance, and blood glucose control. Our data suggest that ZP10A preserves beta-cell function. ZP10A is considered one of the most promising new drug candidates for preventive and therapeutic intervention in type 2 diabetes.[2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following subcutaneous administration, the median Tmax of lixisenatide ranged from 1-3.5 hours, with no clinically relevant differences in the rate of absorption noted between possible injection sites (i.e. thigh, abdomen, or arm). Lixisenatide is presumably eliminated via glomerular filtration and proteolytic degradation. The apparent volume of distribution following subcutaneous administration is approximately 100 L. The mean apparent clearance of lixisenatide is approximately 35 L/h. Metabolism / Metabolites Lixisenatide is likely catabolized via non-specific proteolytic degradation. Biological Half-Life Following the administration of multiple doses in patients with type II diabetes mellitus, the mean terminal half-life of lixisenatide was approximately 3 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In large clinical trials, serum enzyme elevations were no more common with lixisenatide therapy than with placebo or comparator agents. In pooled safety analyses of more than 5000 patients, ALT elevations above 3 times the upper limit of normal occurred in 0.6% of both lixisenatide and placebo groups and no instances of treatment related clinically apparent liver injury were reported. Since licensure, there have been no published case reports of hepatotoxicity due to lixisenatide and the product label does not list liver injury as an adverse event. Thus, liver injury due to lixisenatide, as with other GLP-1 analogues, must be rare, if it occurs at all. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of lixisenatide during breastfeeding. Because lixisenatide is a large peptide molecule with a molecular weight of 4858 daltons, the amount in milk is likely to be very low and absorption is unlikely because it is probably destroyed in the infant's gastrointestinal tract. Until more data become available, lixisenatide should be used with caution during breastfeeding, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Lixisenatide is approximately 55% bound to human plasma proteins. |
| 参考文献 | |
| 其他信息 |
Lixisenatide is a forty-four membered polypeptide consisting of L-His, Gly, L-Glu, Gly, L-Thr, L-Phe, L-Thr, L-Ser, L-Asp, L-Leu, L-Ser, L-Lys, L-Gln, L-Met, L-Glu, L-Glu, L-Glu, L-Ala, L-Val, L-Arg, L-Leu, L-Phe, L-Ile, L-Glu, L-Trp, L-Leu, L-Lys, L-Asn, Gly, Gly, LPro, L-Ser, L-Ser, Gly, L-Ala, L-Pro, L-Pro, L-Ser, L-Lys, L-Lys, L-Lys, L-Lys, L-Lys, and L-Lys-NH2 residues joined in sequence. Used as an adjunct to diet and exercise for the treatment of adults with type II diabetes. It has a role as a glucagon-like peptide-1 receptor agonist, a hypoglycemic agent and a neuroprotective agent. It is a polypeptide and a peptidyl amide.
Lixisenatide is a glucagon-like peptide-1 (GLP-1) receptor agonist used in the treatment of type II diabetes mellitus (T2DM). It is sold by Sanofi-Aventis under the brand name Adlyxin in the US and Lyxumia in the EU. Adlyxin recieved FDA approval July 28, 2016. Lixisenatide is a recombinant DNA produced polypeptide analogue of human glucagon-like peptide-1 (GLP-1) which is used in combination with diet and exercise in the therapy of type 2 diabetes, either alone or in combination with other antidiabetic agents. Therapy with lixisenatide has not been associated with serum enzyme elevations or with episodes of clinically apparent liver injury. See also: Insulin Glargine; Lixisenatide (component of). Drug Indication Lixisenatide is indicated as an adjunct to diet and exercise to improve glycemic control in adult patients with type II diabetes mellitus. It is also available in combination with [insulin glargine] for the same indication. Lyxumia is indicated for the treatment of adults with type 2 diabetes mellitus to achieve glycaemic control in combination with oral glucose lowering medicinal products and/or basal insulin when these, together with diet and exercise, do not provide adequate glycaemic control. , Treatment of type II diabetes mellitus Mechanism of Action The activation of the GLP-1 receptor by lixisenatide results in the activation of adenylyl cyclase. This increases the concentration of cyclic adenosine monophosphate in the cell leading to the activation of protein kinase A (PKA) as well as Epac1 and Epac2. PKA, Epac1, and Epac2 are involved the in release of Ca2+ from the endoplasmic reticulum which is known as the "amplification" pathway which increases insulin release when the triggering pathway is activated. By activating this amplification pathway lixisenatide increases glucose stimulated insulin secretion. Lixisenatide is a once-daily glucagon-like peptide 1 (GLP-1) receptor agonist mimicking several favorable actions of endogenous GLP-1 that result in improved glycemic control with little or no hypoglycemia and weight loss. Phase II trials have shown that lixisenatide 20 μg once daily restores first-phase insulin release in patients with type 2 diabetes and improves the second-phase insulin response. Administered once or twice daily for 4 weeks, it significantly reduced postprandial and fasting blood glucose levels, and glycosylated hemoglobin (HbA(1c)). The efficacy and safety of lixisenatide once daily is being assessed in the GETGOAL Phase III clinical trial program. Results have shown beneficial effects on HbA(1c) compared with placebo in combination with commonly used antidiabetes agents, with no increased risk of hypoglycemia and with beneficial weight reduction. Adverse effects were similar to those observed for available GLP-1 receptor agonists, the most frequent being gastrointestinal. Both GLP-1 receptor agonists and long-acting insulin analogs have demonstrated protective effects on beta cells in preclinical studies. This, along with the pronounced effect of lixisenatide on postprandial plasma glucose, provides a rationale for combining it with long-acting basal insulin analogs, in the hope that the additive effects on glycemic control combined with a potential benefit on islet cells may lead to a new treatment approach to control blood glucose better and prevent long-term complications in patients with type 2 diabetes.[1] The glucagon-like peptide-1 (GLP-1) receptor represents an established therapeutic target in type 2 diabetes mellitus (T2DM). Agents that activate this receptor improve glucose tolerance alongside a low risk of hypoglycaemia, and have the potential to modify disease progression. Lixisenatide is a new potent and selective GLP-1 receptor agonist currently in development. The preclinical pharmacological profile of Lixisenatide suggests actions that are highly relevant to the long-term maintenance of glucose homeostasis. Lixisenatide protected Ins-1 cells (a rat-derived beta-cell line) from both lipid- and cytokine-induced apoptosis. More importantly, Lixisenatide also prevented lipotoxicity-induced insulin depletion in human islets and preserved insulin production, storage and pancreatic beta-cell function in vitro. Enhancement of insulin biosynthesis and pancreatic beta-cell volume could also be demonstrated in animal models of type 2 diabetes. The improvement of glucose-stimulated insulin secretion provided by Lixisenatide occurred in a strictly glucose-dependent manner. In animal models of diabetes, Lixisenatide improved basal blood glucose and HbA(1c) with a rapid onset and sustained duration of action, and prevented the deterioration of pancreatic responsiveness and glucose homeostasis. Lixisenatide also delayed gastric emptying and reduced food intake. The efficacy/safety profile of Lixisenatide is currently being studied further in an extensive ongoing Phase III clinical study programme. This article reviews the preclinical pharmacological profile of Lixisenatide.[3] Introduction: The extent to which postprandial glucagon reductions contribute to lowering of postprandial glucose in patients with type 2 diabetes mellitus (T2DM) is currently unknown. The aim of this analysis was to determine whether a reduction in postprandial glucagon following treatment with the glucagon-like peptide-1 receptor agonist lixisenatide correlates with a reduction in postprandial glucose and glycated hemoglobin (HbA1c) in patients with T2DM. Methods: A post hoc analysis was performed on pooled data from the modified intent-to-treat populations of two lixisenatide Phase 3 trials: GetGoal-M (lixisenatide versus placebo as add-on to metformin) and GetGoal-S (lixisenatide versus placebo as add-on to sulfonylurea [SU] ± metformin). Glucagon levels were assessed 2 h after a standardized meal test performed at baseline and Week 24 and were examined for correlation with changes in 2-h postprandial glucose and HbA1c. Results: Lixisenatide reduced 2-h postprandial glucagon at Week 24 compared with placebo (P < 0.00001). The mean change in postprandial glucagon significantly correlated with reductions in postprandial glucose (P < 0.00001) and HbA1c (P < 0.00001). Conclusion: A reduction in postprandial glucagon following lixisenatide administration correlated with a decrease in postprandial glucose and HbA1c in patients with T2DM insufficiently controlled on metformin and/or SU. This suggests that lowering of postprandial glucagon contributes to the overall glycemic improvement observed with lixisenatide.[4] Objectives: To determine the effects of lixisenatide, a new once-daily (QD) glucagon-like peptide-1 receptor agonist, on postprandial glucose (PPG) and gastric emptying, and the relationship between these effects in patients with type 2 diabetes mellitus (T2DM). Methods: Data were obtained from a randomized, double-blind, placebo-controlled, parallel-group study with treatment duration of 28 days in patients with T2DM receiving ≤2 oral antidiabetic drugs. Lixisenatide was injected subcutaneously using an ascending dose range (5-20 μg) increased every fifth day in increments of 2.5 μg. Blood glucose was determined before and after three standardized meals (breakfast, lunch, and dinner). Gastric emptying of the standardized breakfast was determined by a (13)C-octanoic acid breath test at baseline (Day-1) and at Day 28. Results: A total of 21 and 22 patients were randomized to lixisenatide 20 μg QD and placebo, respectively. With lixisenatide 20 μg QD, there was a reduction in PPG when compared with placebo after breakfast (p<0.0001), lunch (p<0.001) and dinner (p<0.05). Hence, lixisenatide 20 μg administered in the morning exhibited a pharmacodynamic effect on blood glucose throughout the day. Gastric emptying (50% emptying time) increased substantially from baseline with lixisenatide 20 μg QD, but not with placebo (change from baseline ± SD: -24.1 ± 133.1 min for placebo and 211.5 ± 278.5 min for lixisenatide; p<0.01). There was an inverse relationship between PPG area under the curve after breakfast and gastric emptying with lixisenatide 20 μg QD (n=17, r(2)=0.51, p<0.05), but not with placebo. Conclusions: In this study, lixisenatide at a dose of 20 μg QD reduced postprandial glycemic excursions in patients with T2DM, possibly as a result of sustained slowing of gastric emptying.[5] |
| 分子式 |
C215H347N61O65S
|
|---|---|
| 分子量 |
4858.4904282093
|
| 精确质量 |
4857.551
|
| 元素分析 |
C, 53.15; H, 7.20; N, 17.59; O, 21.40; S, 0.66
|
| CAS号 |
1997361-87-1
|
| 相关CAS号 |
Lixisenatide; 320367-13-3
|
| PubChem CID |
16139342
|
| 序列 |
H-His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile-Glu-Trp-Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Ser-Lys-Lys-Lys-Lys-Lys-Lys-NH2; L-histidyl-glycyl-L-alpha-glutamyl-glycyl-L-threonyl-L-phenylalanyl-L-threonyl-L-seryl-L-alpha-aspartyl-L-leucyl-L-seryl-L-lysyl-L-glutaminyl-L-methionyl-L-alpha-glutamyl-L-alpha-glutamyl-L-alpha-glutamyl-L-alanyl-L-valyl-L-arginyl-L-leucyl-L-phenylalanyl-L-isoleucyl-L-alpha-glutamyl-L-tryptophyl-L-leucyl-L-lysyl-L-asparagyl-glycyl-glycyl-L-prolyl-L-seryl-L-seryl-glycyl-L-alanyl-L-prolyl-L-prolyl-L-seryl-L-lysyl-L-lysyl-L-lysyl-L-lysyl-L-lysyl-L-lysinamide
|
| 短序列 |
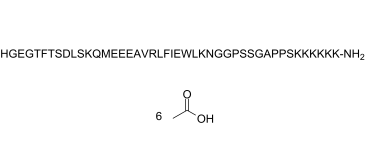
HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPSKKKKKK;
H-HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPSKKKKKK-[NH2]
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
-30.8
|
| tPSA |
2090
|
| 氢键供体(HBD)数目 |
70
|
| 氢键受体(HBA)数目 |
77
|
| 可旋转键数目(RBC) |
170
|
| 重原子数目 |
342
|
| 分子复杂度/Complexity |
11800
|
| 定义原子立体中心数目 |
42
|
| SMILES |
S(C)CC[C@@H](C(N[C@@H](CCC(=O)O)C(N[C@@H](CCC(=O)O)C(N[C@@H](CCC(=O)O)C(N[C@@H](C)C(N[C@H](C(N[C@@H](CCCNC(=N)N)C(N[C@H](C(N[C@@H](CC1C=CC=CC=1)C(N[C@H](C(N[C@@H](CCC(=O)O)C(N[C@@H](CC1=CNC2C=CC=CC1=2)C(N[C@@H](CC(C)C)C(N[C@@H](CCCCN)C(N[C@@H](CC(N)=O)C(NCC(NCC(N1CCC[C@H]1C(N[C@@H](CO)C(N[C@@H](CO)C(NCC(N[C@@H](C)C(N1CCC[C@H]1C(N1CCC[C@H]1C(N[C@H](C(N[C@H](C(N[C@H](C(N[C@H](C(N[C@H](C(N[C@H](C(N[C@H](C(N)=O)CCCCN)=O)CCCCN)=O)CCCCN)=O)CCCCN)=O)CCCCN)=O)CCCCN)=O)CO)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)[C@@H](C)CC)=O)=O)CC(C)C)=O)=O)C(C)C)=O)=O)=O)=O)=O)NC([C@H](CCC(N)=O)NC([C@H](CCCCN)NC([C@H](CO)NC([C@H](CC(C)C)NC([C@H](CC(=O)O)NC([C@H](CO)NC([C@H]([C@@H](C)O)NC([C@H](CC1C=CC=CC=1)NC([C@H]([C@@H](C)O)NC(CNC([C@H](CCC(=O)O)NC(CNC([C@H](CC1=CN=CN1)N)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O
|
| InChi Key |
XVVOERDUTLJJHN-IAEQDCLQSA-N
|
| InChi Code |
InChI=1S/C215H347N61O65S/c1-16-115(10)173(210(337)256-141(68-74-170(299)300)194(321)261-148(94-122-98-232-126-50-24-23-49-124(122)126)199(326)258-143(89-111(2)3)196(323)247-134(58-32-40-83-223)189(316)262-149(96-160(226)285)180(307)235-100-161(286)233-104-165(290)274-85-42-60-156(274)207(334)267-154(108-280)206(333)265-151(105-277)181(308)237-101-162(287)239-117(12)213(340)276-87-44-62-158(276)214(341)275-86-43-61-157(275)208(335)268-153(107-279)204(331)249-132(56-30-38-81-221)187(314)246-131(55-29-37-80-220)186(313)245-130(54-28-36-79-219)185(312)244-129(53-27-35-78-218)184(311)243-128(52-26-34-77-217)183(310)242-127(176(227)303)51-25-33-76-216)272-201(328)146(92-120-45-19-17-20-46-120)260-197(324)144(90-112(4)5)257-190(317)135(59-41-84-231-215(228)229)255-209(336)172(114(8)9)271-177(304)116(11)240-182(309)138(65-71-167(293)294)251-192(319)139(66-72-168(295)296)252-193(320)140(67-73-169(297)298)253-195(322)142(75-88-342-15)254-191(318)137(63-69-159(225)284)250-188(315)133(57-31-39-82-222)248-203(330)152(106-278)266-198(325)145(91-113(6)7)259-200(327)150(97-171(301)302)263-205(332)155(109-281)269-212(339)175(119(14)283)273-202(329)147(93-121-47-21-18-22-48-121)264-211(338)174(118(13)282)270-164(289)103-236-179(306)136(64-70-166(291)292)241-163(288)102-234-178(305)125(224)95-123-99-230-110-238-123/h17-24,45-50,98-99,110-119,125,127-158,172-175,232,277-283H,16,25-44,51-97,100-109,216-224H2,1-15H3,(H2,225,284)(H2,226,285)(H2,227,303)(H,230,238)(H,233,286)(H,234,305)(H,235,307)(H,236,306)(H,237,308)(H,239,287)(H,240,309)(H,241,288)(H,242,310)(H,243,311)(H,244,312)(H,245,313)(H,246,314)(H,247,323)(H,248,330)(H,249,331)(H,250,315)(H,251,319)(H,252,320)(H,253,322)(H,254,318)(H,255,336)(H,256,337)(H,257,317)(H,258,326)(H,259,327)(H,260,324)(H,261,321)(H,262,316)(H,263,332)(H,264,338)(H,265,333)(H,266,325)(H,267,334)(H,268,335)(H,269,339)(H,270,289)(H,271,304)(H,272,328)(H,273,329)(H,291,292)(H,293,294)(H,295,296)(H,297,298)(H,299,300)(H,301,302)(H4,228,229,231)/t115-,116-,117-,118+,119+,125-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,139-,140-,141-,142-,143-,144-,145-,146-,147-,148-,149-,150-,151-,152-,153-,154-,155-,156-,157-,158-,172-,173-,174-,175-/m0/s1
|
| 化学名 |
(4S)-5-[[2-[[(2S,3R)-1-[[(2S)-1-[[(2S,3R)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-5-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S,3S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-4-amino-1-[[2-[[2-[(2S)-2-[[(2S)-1-[[(2S)-1-[[2-[[(2S)-1-[(2S)-2-[(2S)-2-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-6-amino-1-[[(2S)-6-amino-1-[[(2S)-6-amino-1-[[(2S)-6-amino-1-[[(2S)-1,6-diamino-1-oxohexan-2-yl]amino]-1-oxohexan-2-yl]amino]-1-oxohexan-2-yl]amino]-1-oxohexan-2-yl]amino]-1-oxohexan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]carbamoyl]pyrrolidine-1-carbonyl]pyrrolidin-1-yl]-1-oxopropan-2-yl]amino]-2-oxoethyl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]carbamoyl]pyrrolidin-1-yl]-2-oxoethyl]amino]-2-oxoethyl]amino]-1,4-dioxobutan-2-yl]amino]-1-oxohexan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-1-oxopropan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-4-methylsulfanyl-1-oxobutan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-carboxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-2-oxoethyl]amino]-4-[[2-[[(2S)-2-amino-3-(1H-imidazol-5-yl)propanoyl]amino]acetyl]amino]-5-oxopentanoic acid
|
| 别名 |
AVE0010Adlyxin; Lyxumia; ZP10A peptide; Lixisenatide Acetate; 1997361-87-1; Lixisenatide acetate (320367-13-3 free base); ZP10 A peptide; ZP10-A peptide; AVE-0010; AVE 0010
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O: ~50 mg/mL (~9.6 mM)
|
|---|---|
| 溶解度 (体内实验) |
Note: 如何溶解多肽产品?请参考本产品网页右上角“产品说明书”文件,第4页。 配方 1 中的溶解度: 100 mg/mL (19.16 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.2058 mL | 1.0291 mL | 2.0583 mL | |
| 5 mM | 0.0412 mL | 0.2058 mL | 0.4117 mL | |
| 10 mM | 0.0206 mL | 0.1029 mL | 0.2058 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05804513 | Recruiting | Drug: Placebo Drug: Lixisenatide 10 micrograms (50 micrograms/ml in 3 ml) Pen Injector |
Healthy Type 1 Diabetes |
University of Tartu | April 17, 2023 | Phase 4 |
| NCT02020629 | Completed | Drug: Lixisenatide | Type 2 Diabetes | Lund University | December 2013 | Phase 4 |
| NCT02049034 | Completed | Other: Lixisenatide Other: Placebo |
Type 2 Diabetes | University of Surrey | January 2014 | Phase 4 |
| NCT03439943 | Completed | Drug: Lixisenatide Drug: placebo |
Parkinson Disease | University Hospital, Toulouse | June 13, 2018 | Phase 2 |
| NCT02276196 | Completed | Drug: Lixisenatide Drug: Insulin glulisine |
Diabetic Kidney Disease Diabetic Nephropathy |
Amsterdam UMC, location VUmc | September 2014 | Phase 4 |
|