| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg | |||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
| 靶点 |
μ-opioid receptor ( Ki = 3.3 nM ); δ-opioid receptor ( Ki = 48 nM )
μ-Opioid receptor (Ki = 2.3 nM) [3] - δ-Opioid receptor (Ki = 120 nM) [3] - κ-Opioid receptor (Ki = 250 nM) [3] |
|---|---|
| 体外研究 (In Vitro) |
与 delta (Ki = 48 nM) 和 kappa (Ki = 1156 nM) 人阿片受体相比,洛哌丁胺对克隆的 micro (Ki = 3 nM) 人类阿片受体表现出强大的亲和力和选择性。在转染人 mu 阿片受体的中国仓鼠卵巢细胞中,洛哌丁胺有效刺激 [35S]鸟苷-5-O-(3-硫代)三磷酸结合,EC50 为 56 nM,并抑制毛喉素刺激的 cAMP 积累 (IC50 = 25 nM) 。洛哌丁胺可有效抑制爪内注射后福尔马林诱导的后期退缩 (A50 = 6 mg)。 Loperamide 是 CES2 的强抑制剂,K(i) 为 1.5 mM,但仅弱抑制 CES1A1 (IC50 = 0.44 mM)。洛哌丁胺以浓度依赖性方式可逆地阻断高 [K+] 引起的 [Ca2+]i 升高,IC50 为 0.9 mM。 Loperamide (0.1-50 mM) 会产生浓度依赖性的 IBa 峰值降低,IC50 值为 2.5 mM,并且在测试的最高浓度下,在没有任何其他药物的情况下可以完全阻断 IBa。 Loperamide 还可以减弱在 -60 mV 膜电位下记录的 NMDA 诱发电流,IC50 为 73 mM。 Loperamide HCl(R-18553,盐酸洛哌丁胺) 在放射性配体结合实验中对μ阿片受体表现出高亲和力,对δ/κ阿片受体亲和力较低。它竞争性抑制[³H]-二氢吗啡与μ受体结合(Ki=2.3 nM),对δ受体(Ki=120 nM)和κ受体(Ki=250 nM)的亲和力分别低52倍和109倍[3] - 在离体豚鼠回肠制剂中,Loperamide HCl(0.1–10 μM)剂量依赖性抑制电刺激诱导的收缩,EC50为0.8 μM。该效应可被纳洛酮(1 μM)逆转,证实其μ阿片受体介导的活性[3] - 在大鼠结肠上皮细胞中,Loperamide HCl(1–5 μM)通过Gαi偶联受体信号通路抑制肠道液体分泌,5 μM时抑制毛喉素诱导的cAMP积累45%[1] |
| 体内研究 (In Vivo) |
将0.3mg洛哌丁胺注射到发炎的大鼠膝关节的关节内空间,会对膝关节压迫产生强烈的镇痛作用,纳洛酮会拮抗这种镇痛作用,而注射到对侧膝关节或通过肌肉注射的方式则无法抑制压迫引起的血压变化。洛哌丁胺能有效抑制爪内注射后福尔马林诱导的晚期退缩(A50=6微克),但对早期退缩或注射到福尔马林处理爪对侧爪后的退缩无效。局部注射洛哌丁胺也会对弗氏佐剂(ED50=21微克)或胶带剥离(ED50=71微克)诱导的痛觉过敏产生镇痛作用,这可以通过发炎爪子中爪子压力阈值的增加来证明。在所有检查的动物模型中,局部给药后洛哌丁胺的效力与吗啡相当或更好。洛哌丁胺作为一种外周选择性阿片类抗痛觉过敏药物具有潜在的治疗用途,通常没有许多副作用[1]
洛哌丁胺是一种阿片类激动剂,无法穿过血脑屏障,皮下注射时可抑制热痛觉和机械痛觉过敏,局部注射在胫骨肿瘤块上 (7.5-75 mg) 或远处注射到颈毛下方 (4 mg/kg) )在小鼠中。 在蓖麻油诱导的小鼠腹泻模型中,口服Loperamide HCl(0.1、0.3、1 mg/kg)剂量依赖性减少腹泻粪便数量。1 mg/kg剂量较对照组抑制80%的腹泻[4] - 在大鼠炭末肠道转运实验中,Loperamide HCl(0.5 mg/kg,口服)延长肠道转运时间65%,减慢胃肠蠕动[1] - 在吗啡依赖小鼠的戒断模型中,Loperamide HCl(2 mg/kg,腹腔注射)减轻40%的戒断症状(跳跃、舔爪),且未诱导明显的中枢阿片类效应(无镇静或呼吸抑制)[2] |
| 酶活实验 |
在啮齿动物的各种炎性疼痛模型中研究了阿片类止泻剂洛哌丁胺(ADL 2-1294)的抗痛觉过敏特性。与δ(Ki=48nM)和κ(Ki=1156 nM)人阿片受体相比,洛哌丁胺对克隆的微(Ki=3 nM)表现出强烈的亲和力和选择性。洛哌丁胺能有效刺激转染有人μ阿片受体的中国仓鼠卵巢细胞中[35S]鸟苷-5'-O-(3-硫代)三磷酸结合(EC50=56nM),并抑制毛喉素刺激的cAMP积累(IC50=25nM)。[1]
制备富含μ/δ/κ阿片受体的大鼠脑细胞膜悬液,将系列稀释的Loperamide HCl(0.01–1000 nM)与细胞膜悬液、[³H]-二氢吗啡(μ配体)、[³H]-DPDPE(δ配体)或[³H]-U69593(κ配体)在测定缓冲液中混合,25°C孵育90分钟。过滤去除未结合配体,检测放射性强度,采用Cheng-Prusoff方程计算Ki值[3] |
| 细胞实验 |
细胞系:GBM 细胞和小鼠胚胎成纤维细胞 (MEF)
浓度:17.5 µM 孵育时间:1、2、4、6、8、24、30、48 小时 结果:增加了两种细胞系中的主要伴侣 HSPA5。 研究了止泻剂洛哌丁胺对两种培养的海马锥体神经元的高压激活(HVA)钙通道活性和兴奋性氨基酸诱发反应的影响。在负载钙敏感染料fura-2的大鼠海马神经元中,短暂暴露于50 mM含K(+)的培养基[高细胞外钾浓度([K+]o)]引起的细胞内游离钙浓度([Ca2+]i)的升高主要是通过硝苯地平敏感的Ca2+通道介导的,ω-芋螺毒素GVIA(ω-CgTx)敏感的Ca2+通道和对硝苯地平和ω-CGTZ不敏感的通道的贡献较小。洛哌丁胺以浓度依赖的方式可逆地阻断高[K+]o引起的[Ca2+]i升高,IC50为0.9+/-0.2微M。在测试的最高浓度(50微M)下,洛哌丁胺消除了高[K+]o引起的[Ca2+]i升高,否则这一结果仅在无Ca(2+)的培养基中或通过将硝苯地平、ω-CgTx和漏斗网蜘蛛毒液联合应用于含Ca(2+”的培养基来实现。洛哌丁胺的作用既不对纳洛酮敏感,也不被吗啡模仿,其浓度远低于阻断Ca2+通过N-甲基-D-天冬氨酸(NMDA)受体操作的离子载体流入所需的浓度。在全细胞电压钳下培养的小鼠海马锥体神经元也得到了类似的结果。钡离子(IBa)携带的电压激活的Ca2+通道电流在药理学上可分为硝苯地平敏感(L型)和硝苯地平耐药、Ω-CgTx敏感(N型)成分。洛哌丁胺(0.1-50微M)产生峰值IBa的浓度依赖性降低,IC50值为2.5+/-0.4微M,在测试的最高浓度下,可以在没有任何其他药物的情况下完全阻断IBa。洛哌丁胺诱导的阻滞起效和消退迅速,完全可逆,似乎与洛哌丁胺已知的钙调素拮抗作用无关。整个电池IBa的电流-电压特性不受洛哌丁胺的影响,该块不依赖于电压。洛哌丁胺还减弱了在-60 mV膜电位下记录的NMDA诱发电流,IC50为73+/-7微M。NMDA诱发电流的阻断本质上不是竞争性的,不会因细胞外甘氨酸或精胺浓度的升高而逆转,也不受膜保持电位变化的影响。相比之下,红藻氨酸和DL-α-氨基-3-羟基-5-甲基异恶唑丙酸引起的稳态电流相对不受100微摩尔洛哌丁胺的影响。[3] 大鼠结肠上皮细胞接种到24孔板,血清饥饿24小时后,Loperamide HCl(1–5 μM)预处理30分钟,再用毛喉素(10 μM)刺激30分钟。ELISA法定量细胞内cAMP水平,评估分泌抑制效果[1] |
| 动物实验 |
4 mg/kg
Mice Loperamide, an opioid agonist unable to cross the blood-brain barrier, inhibits both thermal and mechanical hyperalgesia when s.c. injected, locally over the tibial tumoral mass (7.5-75 microg) or distantly, under the fur of the neck (4 mg/kg). These analgesic effects seem peripherally mediated since they are reverted by the administration of naloxone methiodide (10 mg/kg) and because the withdrawal latencies of the contralateral, non-affected, paws remain unaltered. Furthermore, only cyprodime (1 mg/kg) but not naltrindole (0.1 mg/kg) or nor-binaltorphimine (10 mg/kg) blocked these effects, showing the involvement of gamma-opioid receptors in the peripheral analgesia induced by loperamide on thermal and mechanical hyperalgesia. The advantages of using peripheral acting opiates -- devoid of central colateral effects -- for the treatment of cancer related pain are suggested.[4] Mouse Castor Oil-Induced Diarrhea Model: Male ICR mice were randomly divided into control (saline) and Loperamide HCl groups (0.1, 0.3, 1 mg/kg, p.o., n=8 per group). Castor oil (0.1 mL/10 g) was administered 30 minutes after drug treatment. The number of diarrheal stools was counted every 30 minutes for 4 hours [4] - Rat Intestinal Transit Model: Male Wistar rats were fasted for 18 hours, then administered Loperamide HCl (0.5 mg/kg, p.o.) or saline. Thirty minutes later, a charcoal meal (1 mL/100 g) was gavaged. Rats were sacrificed 1 hour later, and the distance traveled by charcoal in the small intestine was measured to calculate transit time [1] - Mouse Morphine Withdrawal Model: Male Swiss mice were rendered morphine-dependent by subcutaneous injection of morphine (20 mg/kg/day) for 7 days. On day 8, Loperamide HCl (2 mg/kg, i.p.) or saline was administered 30 minutes before naloxone (1 mg/kg, i.p.) to precipitate withdrawal. Withdrawal signs (jumping, paw licking, diarrhea) were scored for 30 minutes [2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Loperamide is well absorbed from the gastrointestinal tract; however, it undergoes extensive first-pass metabolism to form metabolites that are excreted in the bile. Therefore, little loperamide actually reaches the systemic circulation. The drug bioavailability is less than 1%. Following oral administration of a 2 mg capsule of loperamide, plasma concentrations of unchanged drug were below 2 ng/mL. Plasma loperamide concentrations are highest approximately five hours after administration of an oral capsule of loperamide and 2.5 hours after the liquid formulation of the drug. Loperamide and its metabolites in the systemic circulation undergo biliary excretion. Excretion of the unchanged loperamide and its metabolites mainly occurs through the feces. Only 1% of an absorbed dose excreted unchanged in the urine. Loperamide has a large volume of distribution. Although highly lipophilic, loperamide does not cross the blood-brain barrier and generally acts peripherally. Tritium-labelled loperamide was administered orally to eight groups of five fasted male Wistar rats (250 +/- 10 g) at a dosage of 1.25 mg/kg. Urine and feces were collected for up to 4 days. The rats were killed at different times from 1 to 96 hours after drug administration in order to examine blood, organs and tissues. In one rat, the bile was cannulated for 48 hours. The radioactive content of each sample was measured and the fractions due to loperamide, metabolites, and volatile radioactivity were determined by the inverse isotope dilution technique and lyophilization. Only 5% of the drug and its metabolites was recovered from the urine, the bulk being excreted with the feces. Drug plasma levels were low at all times. Maximum plasma levels of unchanged loperamide did not exceed 0.22% of the administered dose corresponding to about 75 mg/mL of plasma. The gastrointestinal tract contained about 85% of loperamide 1 hour after dosing. Brain levels were extremely low, never exceeding 22 ng/g brain tissue, or 0.005% of the administered dose. The existence of an enterohepatic shunt was shown, but the uptake of the drug into the general circulation was low. Differentiation between total radioactivity and nonvolatile radioactivity demonstrated that most of the residual organ radioactivity was due to tritiated water. Three male volunteers received orally 2.0 mg of 3H-loperamide (specific activity 64 mCi/mM) in gelatine capsules. Control samples of blood, urine and feces were obtained before administration. Blood was collected on heparin 1, 2, 4, 8, 24, 72 and 168 hours thereafter. Urine was collected for seven days and feces for eight days. The radioactive content of each sample was measured and the fractions due to loperamide, metabolites and volatile radioactivity were determined by the inverse isotope dilution technique and lyophilization. The fate of orally administered 3H-loperamide in man appeared to be similar to that in rats. The peak plasma level of loperamide occurred 4 hours after treatment and was less than 2 ng/mL or about 0.3% of the administered dose. About 1% of the administered dose was excreted unaltered with the urine and 6% as nonvolatile metabolites. About 40% of the administered dose was excreted with the feces, mainly within the first four days; 30% of this amount was due to unchanged drug. Studies on distribution in rats show a high affinity for the gut wall with a preference for binding to receptors of the longitudinal muscle layer. The plasma protein binding of loperamide is 95%, mainly to albumin. Non-clinical data have shown that loperamide is a P-glycoprotein substrate. /MILK/ Small amounts of loperamide may appear in human breast milk. For more Absorption, Distribution and Excretion (Complete) data for Loperamide (8 total), please visit the HSDB record page. Metabolism / Metabolites Loperamide is extensively metabolized. The primary metabolic pathway is oxidative N-demethylation mediated by CYP2C8 and CYP3A4, to form N-demethyl loperamide. CYP2B6 and CYP2D6 play a minor role in loperamide N-demethylation. Metabolites of loperamide are pharmacologically inactive. Loperamide is almost completely extracted by the liver, where it is predominantly metabolized, conjugated and excreted via the bile. Oxidative N-demethylation is the main metabolic pathway for loperamide, and is mediated mainly through CYP3A4 and CYP2C8. Due to this very high first pass effect, plasma concentrations of unchanged drug remain extremely low. In contrast with the Parkinson's-like effects associated with the mitochondrial neurotoxin N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and the neuroleptic agent haloperidol, there exist no reports on adverse central nervous system (CNS) effects with the structurally related N-substituted-4-arylpiperidin-4-ol derivative and antidiarrheal agent loperamide. Although this difference can be attributed to loperamide's P-glycoprotein substrate properties that prevent it from accessing the brain, an alternative possibility is that loperamide metabolism in humans is different from that of MPTP and haloperidol and does not involve bioactivation to a neurotoxic pyridinium species. In the current study, loperamide bioactivation was examined with particular focus on identification of pyridinium metabolites. A NADPH-dependent disappearance of loperamide was observed in both rat and human liver microsomes (human t(1/2) = 13 min; rat t(1/2) = 22 min). Loperamide metabolism was similar in human and rat and involved N-dealkylation to N-desmethylloperamide (M3) as the principal metabolic fate. Other routes of loperamide biotransformation included N- and C-hydroxylation to the loperamide-N-oxide (M4) and carbinolamide (M2) metabolites, respectively. Furthermore, the formation of an additional metabolite (M5) was also discernible in human and rat liver microsomes. The structure of M5 was assigned to the pyridinium species (LPP(+)) based on comparison of the liquid chromatography/tandem mass spectrometry characteristics to the pyridinium obtained from loperamide via a chemical reaction. Loperamide metabolism in human microsomes was sensitive to ketoconazole and bupropion treatment, suggesting P4503A4 and -2B6 involvement. Recombinant P4503A4 catalyzed all of the loperamide biotransformation pathways in human liver microsomes, whereas P4502B6 was only responsible for N-dealkylation and N-oxidation routes. The wide safety margin of loperamide (compared with MPTP and haloperidol) despite metabolism to a potentially neurotoxic pyridinium species likely stems from a combination of factors that include a therapeutic regimen normally restricted to a few days and the fact that loperamide and perhaps LPP(+) are P-glycoprotein substrates and are denied entry into the CNS. The differences in safety profile of haloperidol and loperamide despite a common bioactivation event supports the notion that not all compounds undergoing bioactivation in vitro will necessarily elicit a toxicological response in vivo. Loperamide has known human metabolites that include N-Desmethyloperamide. Biological Half-Life The apparent elimination half-life of loperamide is 10.8 hours with a range of 9.1 to 14.4 hours. The apparent elimination half-life of loperamide in healthy adults is 10.8 hours (range 9.1-14.4 hours). The oral bioavailability of Loperamide HCl in humans is 40% [1] - After oral administration of 4 mg in humans, the maximum plasma concentration (Cmax) is 2.5 ng/mL, achieved at 2.5 hours (Tmax), and the plasma half-life (t1/2) is 10.8 hours [1] - It is highly bound to human plasma proteins (97–99%) [3] - Loperamide HCl does not cross the blood-brain barrier significantly at therapeutic doses, due to efflux by P-glycoprotein [2] - Metabolism occurs primarily in the liver via cytochrome P450 3A4 (CYP3A4) and CYP2C8. Approximately 70% of the dose is excreted in feces and 30% in urine, mostly as metabolites [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Loperamide is a solid. Loperamide is used in the control and symptomatic relief of acute nonspecific diarrhea and of chronic diarrhea associated with inflammatory bowel disease. HUMAN EXPOSURE AND TOXICITY: Loperamide is an over-the-counter antidiarrheal with mu-opioid agonist activity. Central nervous system opioid effects are not observed after therapeutic oral dosing because of poor bioavailability and minimal central nervous system penetration. However, central nervous system opioid effects do occur after supratherapeutic oral doses. Oral loperamide abuse as an opioid substitute has been seen among patients attempting to self-treat their opioid addiction. Ventricular dysrhythmias and prolongation of the QRS duration and QTc interval have been reported after oral loperamide abuse. In postmarketing experiences, paralytic ileus associated with abdominal distention has been reported rarely. Most of these cases occurred in patients with acute dysentery, following overdosage of the drug, or in children younger than 2 years of age. ANIMAL STUDIES: Loperamide administration significantly suppressed foraging behavior in rats and reduced their body weight. The intravenous injection of loperamide induced an immediate fall in blood pressure and heart rate in anesthetized rats. In a study in rats using loperamide dosages up to 133 times the maximum human dosage (on a mg/kg basis) for 18 months, there was no evidence of carcinogenicity. Beagle dogs were given loperamide in gelatin capsules at 5.0, 1.25 and 0.31 mg/kg six days a week for 12 months. Some depression was seen during the first week of drug administration at 1.25 and 5 mg/kg. Behavior and appearance were normal during the rest of the experiment, except that hemorrhagic stools were seen from time to time at 5 mg/kg and soft stools at 0.31 and 1.25 mg/kg, especially during the first 6 weeks of drug administration. Pregnant primiparous female rats were given loperamide in their diet at 40, 10 and 2.5 mg/100 g of food from day 6 through day 15 of pregnancy. On day 22, fetuses were delivered by caesarean section. At 40 mg/100 g food, only 1 female out of 20 became pregnant. There was no significant difference between the control group and the 2.5 and 10 mg/100 g food-dosed groups in pregnancy rate; number of implantations per dam; litter size, percentage of live, dead and resorbed fetuses; distribution of live, dead and resorbed fetuses in the left and right uterine horns; and body weight of live young. No macroscopic, visceral, or skeletal malformations were seen. Results of in vivo and in vitro studies carried out indicated that loperamide is not genotoxic. Hepatotoxicity As with most opiates in current use, therapy with loperamide has not been linked to serum enzyme elevations. There have been no convincing cases of idiosyncratic acute, clinically apparent liver injury attributed to either agent. The reason for its lack of hepatotoxicity may relate to the low doses used and lack of significant systemic absorption. What loperamide is absorbed is metabolized in the liver. References on the safety and potential hepatotoxicity of loperamide are given in the overview section of the Opioids. Last updated: 20 May 2019 Drug Class: Gastrointestinal Agents; Opioids Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation The amount of loperamide that enters milk from a prodrug of loperamide is minimal. Use of loperamide during breastfeeding is unlikely to affect the infant with standard doses. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Based on literature information, the plasma protein binding of loperamide is about 95%. Interactions Non-clinical data have shown that loperamide is a P-glycoprotein substrate. Concomitant administration of loperamide (16 mg single dose) with quinidine, or ritonavir, which are both Pglycoprotein inhibitors, resulted in a 2 to 3-fold increase in loperamide plasma levels. The clinical relevance of this pharmacokinetic interaction with P-glycoprotein inhibitors, when loperamide is given at recommended dosages is unknown. Concomitant treatment /of loperamide/ with oral desmopressin resulted in a 3-fold increase of desmopressin plasma concentrations, presumably due to slower gastrointestinal motility. Loperamide is biotransformed in vitro by the cytochromes P450 (CYP) 2C8 and 3A4 and is a substrate of the P-glycoprotein efflux transporter. Our aim was to investigate the effects of itraconazole, an inhibitor of CYP3A4 and P-glycoprotein, and gemfibrozil, an inhibitor of CYP2C8, on the pharmacokinetics of loperamide. In a randomized crossover study with 4 phases, 12 healthy volunteers took 100 mg itraconazole (first dose 200 mg), 600 mg gemfibrozil, both itraconazole and gemfibrozil, or placebo, twice daily for 5 days. On day 3, they ingested a single 4-mg dose of loperamide. Loperamide and N-desmethylloperamide concentrations in plasma were measured for up to 72 hr and in urine for up to 48 hr. Possible central nervous system effects of loperamide were assessed by the Digit Symbol Substitution Test and by subjective drowsiness. Itraconazole raised the peak plasma loperamide concentration (Cmax) 2.9-fold (range, 1.2-5.0; p < 0.001) and the total area under the plasma loperamide concentration-time curve (AUC(0-infinity)) 3.8-fold (1.4-6.6; p < 0.001) and prolonged the elimination half-life (t(1/2)) of loperamide from 11.9 to 18.7 hr (p < 0.001). Gemfibrozil raised the Cmax of loperamide 1.6-fold (0.9-3.2; P < 0.05) and its AUC(0-infinity) 2.2-fold (1.0-3.7; P < 0.05) and prolonged its t(1/2) to 16.7 hr (P < 0.01). The combination of itraconazole and gemfibrozil raised the Cmax of loperamide 4.2-fold (1.5-8.7; P < 0.001) and its AUC(0-infinity) 12.6-fold (4.3-21.8; P < 0.001) and prolonged the t(1/2) of loperamide to 36.9 hr (p < 0.001). The amount of loperamide excreted into urine within 48 hr was increased 3.0-fold, 1.4-fold and 5.3-fold by itraconazole, gemfibrozil and their combination, respectively (p < 0.05). Itraconazole, gemfibrozil and their combination reduced the plasma AUC(0-72) ratio of N-desmethylloperamide to loperamide by 65%, 46% and 88%, respectively (p < 0.001). No significant differences were seen in the Digit Symbol Substitution Test or subjective drowsiness between the phases. Itraconazole, gemfibrozil and their combination markedly raise the plasma concentrations of loperamide. Although not seen in the psychomotor tests used, an increased risk of adverse effects should be considered during concomitant use of loperamide with itraconazole, gemfibrozil and especially their combination. Non-Human Toxicity Values LD50 Dog iv 2.8 mg/kg LD50 Dog oral >40 mg/kg LD50 Guinea pig oral 41.5 mg/kg LD50 Rat (young, female) oral 261 mg/kg For more Non-Human Toxicity Values (Complete) data for Loperamide (10 total), please visit the HSDB record page. The oral LD50 of Loperamide HCl in mice is 135 mg/kg, and in rats is 240 mg/kg [4] - Common clinical adverse effects include constipation (15% of patients), abdominal cramps (8%), and bloating (5%), which are mild and reversible [1][4] - No significant hepatotoxicity or nephrotoxicity was observed in long-term clinical use, with serum liver enzymes and renal function parameters within normal ranges [1] - Concurrent use with CYP3A4 inhibitors (e.g., erythromycin, ketoconazole) or P-glycoprotein inhibitors (e.g., quinidine) increases plasma concentrations, potentially enhancing central opioid effects (rare cases of sedation or respiratory depression) [2] |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Antidiarrheals /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Loperamide is included in the database. Loperamide is used in the control and symptomatic relief of acute nonspecific diarrhea and of chronic diarrhea associated with inflammatory bowel disease. /Included in US product label/ The fixed combination containing loperamide and simethicone is used for the control and symptomatic relief of diarrhea when relief of flatulence, bloating, and gas pain also is indicated. /Included in US product label/ For more Therapeutic Uses (Complete) data for Loperamide (7 total), please visit the HSDB record page. Drug Warnings Loperamide is generally well tolerated; however, abdominal pain, distention or discomfort, constipation, drowsiness, dizziness, fatigue, dry mouth, nausea and vomiting, and epigastric pain may occur. Children may be more sensitive to adverse CNS effects of the drug than adults. Hypersensitivity reactions including rash have been reported. Adverse effects of loperamide are difficult to distinguish from symptoms associated with the diarrheal syndrome, but adverse GI effects are reported to be less frequent after administration of loperamide than after administration of diphenoxylate with atropine. In postmarketing experiences, paralytic ileus associated with abdominal distention has been reported rarely. Most of these cases occurred in patients with acute dysentery, following overdosage of the drug, or in children younger than 2 years of age. Safety and efficacy of loperamide in children younger than 2 years of age have not been established. Loperamide should be used with particular caution in young children because of the greater variability of response in this age group. The presence of dehydration, especially in younger children, may further influence the variability of response to the drug. Loperamide should not be used in the treatment of diarrhea resulting from some infections or in patients with pseudomembranous colitis (e.g., associated with antibiotics). Loperamide is contraindicated in patients with a known hypersensitivity to the drug and in patients in whom constipation must be avoided. Patients receiving loperamide should be advised to consult their clinician if the diarrhea persists for longer than 2 days, if symptoms worsen, if abdominal swelling or bulging develops, or if fever develops. For self-medication, loperamide should not be used for longer than 2 days unless directed by a clinician. Loperamide should also not be used for self-medication if diarrhea is accompanied by high fever (greater than 38.3 °C), if blood is present in the stool, or if rash or other allergic reaction to the drug has occurred previously. If a patient is receiving an anti-infective or has a history of liver disease, a physician should be consulted before the drug is used for self-medication. For more Drug Warnings (Complete) data for Loperamide (12 total), please visit the HSDB record page. Pharmacodynamics Loperamide is an anti-diarrheal agent that provides symptomatic relief of diarrhea. It decreases peristalsis and fluid secretion in the gastrointestinal tract, delays colonic transit time, and increases the absorption of fluids and electrolytes from the gastrointestinal tract. Loperamide also increases rectal tone, reduces daily fecal volume, and increases the viscosity and bulk density of feces. It also increases the tone of the anal sphincter, thereby reducing incontinence and urgency. The onset of action is about one hour and the duration of action can be up to three days. While loperamide is a potent mu-opioid receptor agonist, it does not mediate significant analgesic activity at therapeutic and supratherapeutic doses. However, at high doses of loperamide, inhibition of P-glycoprotein-mediated drug efflux may allow loperamide to cross the blood-brain barrier, where loperamide can exert central opioid effects and toxicity. At very high plasma concentrations, loperamide can interfere with cardiac conduction. Because loperamide inhibits the Na + -gated cardiac channels and ether-a-go-go–related gene potassium channels, the drug can prolong the QRS complex and the QTc interval, which can lead to ventricular dysrhythmias, monomorphic and polymorphic ventricular tachycardia, torsade de pointes, ventricular fibrillation, Brugada syndrome, cardiac arrest, and death. Loperamide HCl (R-18553) is a peripherally acting μ-opioid receptor agonist [1][2][3][4] - Its primary mechanism of action involves activating μ-opioid receptors in the gastrointestinal tract, inhibiting acetylcholine release, reducing intestinal peristalsis, and suppressing fluid secretion, thereby exerting an antidiarrheal effect [1][3] - Clinical indications include acute diarrhea (traveler’s diarrhea, acute infectious diarrhea) and chronic diarrhea (inflammatory bowel disease, irritable bowel syndrome-related diarrhea) [1][4] - The lack of significant central nervous system effects at therapeutic doses is due to poor blood-brain barrier penetration, minimizing the risk of addiction and respiratory depression [2][3] - Clinical doses range from 2 mg to 16 mg daily, administered orally (initial dose 4 mg, subsequent 2 mg after each loose stool, not exceeding 16 mg/day in adults) [1][4] |
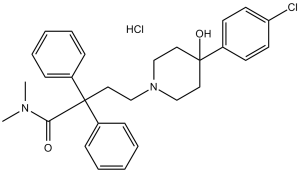
| 分子式 |
C29H34CL2N2O2
|
|
|---|---|---|
| 分子量 |
513.5
|
|
| 精确质量 |
512.199
|
|
| 元素分析 |
C, 67.83; H, 6.67; Cl, 13.81; N, 5.46; O, 6.23
|
|
| CAS号 |
34552-83-5
|
|
| 相关CAS号 |
Loperamide-d6 hydrochloride; 1189469-46-2; 34552-83-5 (HCl); 53179-11-6 (free)
|
|
| PubChem CID |
3955
|
|
| 外观&性状 |
White to light yellow solid powder
|
|
| 沸点 |
647.2ºC at 760 mmHg
|
|
| 熔点 |
223-225°C
|
|
| 闪点 |
345.2ºC
|
|
| LogP |
5.827
|
|
| tPSA |
43.78
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
7
|
|
| 重原子数目 |
34
|
|
| 分子复杂度/Complexity |
623
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
ClC1C([H])=C([H])C(=C([H])C=1[H])C1(C([H])([H])C([H])([H])N(C([H])([H])C([H])([H])C(C(N(C([H])([H])[H])C([H])([H])[H])=O)(C2C([H])=C([H])C([H])=C([H])C=2[H])C2C([H])=C([H])C([H])=C([H])C=2[H])C([H])([H])C1([H])[H])O[H]
|
|
| InChi Key |
PGYPOBZJRVSMDS-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C29H33ClN2O2.ClH/c1-31(2)27(33)29(24-9-5-3-6-10-24,25-11-7-4-8-12-25)19-22-32-20-17-28(34,18-21-32)23-13-15-26(30)16-14-23;/h3-16,34H,17-22H2,1-2H3;1H
|
|
| 化学名 |
4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]-N,N-dimethyl-2,2-diphenylbutanamide;hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.87 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.87 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.87 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 5%DMSO + Corn oil: 7.5mg/ml (14.61mM) 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9474 mL | 9.7371 mL | 19.4742 mL | |
| 5 mM | 0.3895 mL | 1.9474 mL | 3.8948 mL | |
| 10 mM | 0.1947 mL | 0.9737 mL | 1.9474 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04823065 | Recruiting | Drug: Loperamide Pill Drug: hyoscine-n-butylbromide |
Endometrial Cancer Ovarian Cancer |
Centre de recherche du Centre hospitalier universitaire de Sherbrooke |
September 1, 2018 | Phase 1 Phase 2 |
| NCT05252988 | Recruiting | Drug: Neratinib Drug: Loperamide Drug: Colesevelam |
Early-stage Breast Cancer HER2 Positive Breast Cancer Hormone Receptor Positive |
Spanish Breast Cancer Research Group |
August 31, 2022 | Phase 2 |
| NCT05520723 | Recruiting | Drug: Sacituzumab govitecan Drug: Loperamide |
Triple Negative Breast Cancer Breast Cancer |
MedSIR | February 7, 2023 | Phase 2 |
| NCT05677282 | Recruiting | Drug: Loperamide Drug: Rifaximin 550 MG Drug: Azithromycin 500 MG |
Diarrhoea;Acute Diarrhea Travelers |
Henry M. Jackson Foundation for the Advancement of Military Medicine |
October 28, 2022 | Phase 4 |
| NCT04186936 | Completed | Drug: Loperamide HCl Drug: Simethicone |
Healthy | McNeil AB | December 5, 2019 | Phase 1 |