| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
HIV protease (Ki = 1.3 pM)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:洛匹那韦与突变型 HIV 蛋白酶(V82A、V82F 和 V82T)结合,Ki 分别为 4.9 pM、3.7 pM 和 3.6 pM。 0.5 nM 的洛匹那韦可抑制 93% 的野生型 HIV 蛋白酶活性。在 MT4 细胞中,在不存在和存在 50% HS 的情况下,Lopinavir 均抑制 HIV 蛋白酶活性,EC50 分别为 17 nM 和 102 nM。洛匹那韦在肝微粒体中以 NADPH 依赖性方式转化为多种代谢物,主要代谢物为 M-3 和 M-4。 Lopinavir 是 Caco-2 单层中 Rh123 外流的有效抑制剂,IC50 为 1.7 mM。 LS 180V 细胞中洛匹那韦暴露(72 小时)会降低细胞内 Rh123 的含量。洛匹那韦在 LS 180V 细胞中诱导 P-糖蛋白免疫反应蛋白和信使 RNA 水平。 Lopinavir 抑制 C 亚型克隆 C6,IC50 为 9.4 nM。 Lopinavir 在人肝微粒体中抑制 CYP3A,IC50 为 7.3 mM,同时对人 CYP1A2、2B6、2C9、2C19 和 2D6 产生可忽略或微弱的抑制。激酶测定:洛匹那韦是一种有效的 HIV 蛋白酶抑制剂,Ki 为 1.3 pM。目标:HIV 蛋白酶 Lopinavir 是 Caco-2 单层中 Rh123 外流的有效抑制剂,IC50 为 1.7 mM。细胞测定:LS 180V 细胞中洛匹那韦暴露(72 小时)会降低细胞内 Rh123 的含量。洛匹那韦在 LS 180V 细胞中诱导 P-糖蛋白免疫反应蛋白和信使 RNA 水平。 Lopinavir 抑制 C 亚型克隆 C6,IC50 为 9.4 nM。 Lopinavir 在人肝微粒体中抑制 CYP3A,IC50 为 7.3 mM,同时对人 CYP1A2、2B6、2C9、2C19 和 2D6 产生可忽略或微弱的抑制。
|
||
| 体内研究 (In Vivo) |
洛匹那韦(10 mg/kg,口服)在大鼠中的 Cmax 为 0.8 μg/mL,口服生物利用度为 25%。
|
||
| 酶活实验 |
洛匹那韦是一种有效的 HIV 蛋白酶抑制剂,Ki 为 1.3 pM。磷脂 HIV IC50 为 1.7 mM 表明洛匹那韦是 Caco-2 单层中 Rh123 外流的强抑制剂。
|
||
| 细胞实验 |
在 LS 180V 细胞中,洛匹那韦暴露(72 小时)会降低细胞内 Rh123 的量。在 LS 180V 细胞中,洛匹那韦可增加信使 RNA 和 P-糖蛋白免疫反应蛋白的水平。洛匹那韦抑制 C 亚型克隆 C6 的 IC50 为 9.4 nM。在人肝微粒体中,洛匹那韦抑制 CYP3A,IC50 为 7.3 mM,但几乎不抑制人 CYP1A2、2B6、2C9、2C19 和 2D6。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
When administered alone, lopinavir has exceptionally low oral bioavailability (~25%) - for this reason, it is exclusively co-administered with ritonavir, which dramatically improves bioavailability, hinders drug metabolism, and allows for the attainment of therapeutic lopinavir concentrations. Following oral administration of lopinavir/ritonavir, maximal plasma concentrations are achieved at approximately 4.4 hours (Tmax), and the Cmax and AUCtau are 9.8 ± 3.7 - 11.8 ± 3.7 µg/mL and 92.6 ± 36.7 - 154.1 ± 61.4 μg•h/mL, respectively. Relative to administration in the fasted state, administration with a meal increases the AUC of the tablet formulation slightly (~19%) but dramatically increases the AUC of the oral solution formulation (~130%). Lopinavir is primarily eliminated in the feces. Following oral administration, approximately 10.4 ± 2.3% of the administered dose is excreted in the urine and 82.6 ± 2.5% is excreted in the feces. Unchanged parent drug accounted for 2.2% and 19.8% of the administered dose in urine and feces, respectively. The volume of distribution of lopinavir following oral administration is approximately 16.9 L. The estimated apparent clearance following oral administration is approximately 6-7 L/h. At steady state, lopinavir is approximately 98-99% bound to plasma proteins. Lopinavir binds to both alpha-1-acid glycoprotein (AAG) and albumin; however, it has a higher affinity for AAG. At steady state, lopinavir protein binding remains constant over the range of observed concentrations after 400/100 mg KALETRA twice daily, and is similar between healthy volunteers and HIV-1 positive patients. In a pharmacokinetic study in HIV-1 positive subjects (n = 19), multiple dosing with 400/100 mg KALETRA twice daily with food for 3 weeks produced a mean SD lopinavir peak plasma concentration (Cmax) of 9.8 + or - 3.7 ug/mL, occurring approximately 4 hours after administration. The mean steady-state trough concentration prior to the morning dose was 7.1 + or - 2.9 ug/mL and minimum concentration within a dosing interval was 5.5 + or - 2.7 ug/mL. Lopinavir AUC over a 12 hour dosing interval averaged 92.6 + or - 36.7 ug*h/mL. The absolute bioavailability of lopinavir co-formulated with ritonavir in humans has not been established. Under nonfasting conditions (500 kcal, 25% from fat), lopinavir concentrations were similar following administration of KALETRA co-formulated capsules and oral solution. When administered under fasting conditions, both the mean AUC and Cmax of lopinavir were 22% lower for the KALETRA oral solution relative to the capsule formulation. Lopinavir and ritonavir are distributed into milk in rats; it is not known whether the drugs are distributed into human milk. The pharmacokinetics of once daily Kaletra have been evaluated in HIV-1 infected subjects naive to antiretroviral treatment. Kaletra 800/200 mg was administered in combination with emtricitabine 200 mg and tenofovir DF 300 mg as part of a once daily regimen. Multiple dosing of 800/200 mg Kaletra once daily for 4 weeks with food (n = 24) produced a mean + or - 3.7 SD lopinavir peak plasma concentration (Cmax) of 11.8 + or - 3.7 ug/mL, occurring approximately 6 hours after administration. The mean steady-state lopinavir trough concentration prior to the morning dose was 3.2 + or - 3.7 2.1 ug/mL and minimum concentration within a dosing interval was 1.7 + or - 3.7 1.6 ug/mL. Lopinavir AUC over a 24 hour dosing interval averaged 154.1 + or - 3.7 61.4 ug* h/mL. For more Absorption, Distribution and Excretion (Complete) data for Lopinavir (11 total), please visit the HSDB record page. Metabolism / Metabolites Lopinavir undergoes extensive oxidative metabolism, almost exclusively via hepatic CYP3A isozymes. Co-administration with ritonavir, a potent inhibitor of CYP3A enzymes, helps to stave off lopinavir's biotransformation and increase plasma levels of active antiviral drug. Twelve metabolites have been identified _in vitro_, with the C-4 oxidation products M1, M3, and M4 being the predominant metabolites found in plasma. The structures of these primary metabolites have been identified, but precise structural information regarding the remaining minor metabolites has not been elucidated. Lopinavir was metabolised in rat, dog and human primarily by hepatic CYP3A4 isoenzymes. Radioactivity in rat and dog faeces consisted largely of unchanged parent compound after oral administration. Although there were similarities in metabolite pattern between rat, dog and human, qualitative and quantitative differences were observed. The metabolism of lopinavir was sensitive to inhibition of ritonavir, which is in accordance with the inhibition of metabolic clearance of lopinavir by ritonavir observed in the rat. In vitro experiments with human hepatic microsomes indicate that lopinavir primarily undergoes oxidative metabolism. Lopinavir is extensively metabolized by the hepatic cytochrome P450 system, almost exclusively by the CYP3A isozyme. Ritonavir is a potent CYP3A inhibitor which inhibits the metabolism of lopinavir, and therefore increases plasma levels of lopinavir. A (14)C-lopinavir study in humans showed that 89% of the plasma radioactivity after a single 400/100 mg Kaletra dose was due to parent drug. At least 13 lopinavir oxidative metabolites have been identified in man. Ritonavir has been shown to induce metabolic enzymes, resulting in the induction of its own metabolism. Pre-dose lopinavir concentrations decline with time during multiple dosing, stabilizing after approximately 10 to 16 days. Biological Half-Life The elimination half-life of lopinavir is 6.9 ± 2.2 hours. After single dose administration, mean elimination half-life ranged between 2 to 3 hours and seemed to be increased after multiple dose administration (about 4-6 hr). |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Some degree of serum aminotransferase elevations occur in a high proportion of patients taking lopinavir containing antiretroviral regimens. Moderate-to-severe elevations in serum aminotransferase levels (>5 times the upper limit of normal) are found in 3% to 10% of patients, although rates may be higher in patients with HIV-HCV coinfection. These elevations are usually asymptomatic and self-limited and can resolve even with continuation of the medication. Clinically apparent liver disease due to lopinavir/ritonavir occurs, but is rare. The latency to onset of symptoms or jaundice is usually 1 to 8 weeks and the pattern of serum enzyme elevations varies from hepatocellular to cholestatic or mixed. The injury is usually self-limited; however, fatal cases have been reported. In addition, initiation of lopinavir/ritonavir based highly active antiretroviral therapy can lead to exacerbation of an underlying chronic hepatitis B or C in coinfected individuals, typically arising 2 to 12 months after starting therapy, and associated with a hepatocellular pattern of serum enzyme elevations and increases in serum levels of hepatitis B virus (HBV) DNA or hepatitis C virus (HCV) RNA. Lopinavir therapy has not been clearly linked to lactic acidosis and acute fatty liver that is reported in association with several nucleoside analogue reverse transcriptase inhibitors. Likelihood score: D (possible, rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Lopinavir appears in breastmilk in low levels and can be found in the serum of some breastfed infants. Although lopinavir has been associated with impaired adrenal gland function when given directly to infants, the effect is dose related. No adverse infant effects have been clearly caused by the small amounts of lopinavir in breastmilk. Achieving and maintaining viral suppression with antiretroviral therapy decreases breastfeeding transmission risk to less than 1%, but not zero. Individuals with HIV who are on antiretroviral therapy with a sustained undetectable viral load and who choose to breastfeed should be supported in this decision. If a viral load is not suppressed, banked pasteurized donor milk or formula is recommended. Ritonavir used as a booster has been studied in several studies of breastfeeding mothers. It is excreted into milk in measurable concentrations and low levels can be found in the blood of some breastfed infants. No reports of adverse reactions in breastfed infants have been reported. For more information, refer to the LactMed record on ritonavir. ◉ Effects in Breastfed Infants A study compared the rates of severe anemia in 3 groups of infants who received postpartum prophylaxis with zidovudine for prevention of maternal-to-child transmission of HIV infection. Through 6 months of age, breastfed infants whose mothers received HAART had a higher rate of severe anemia (7.4%) than breastfed infants whose mothers received only zidovudine (5.3%). Formula-fed infants had the lowest rate of severe anemia (2.5%). The anemia generally responded well to iron and multivitamin supplementation, and discontinuation of zidovudine. An unblinded study in Uganda compared the outcomes of breastfed infants and their HIV-positive mothers who were randomized to receive antiretroviral therapy that was based either on efavirenz 600 mg once daily or lopinavir 400 mg plus ritonavir 100 mg twice daily during breastfeeding. All mothers received lamivudine 150 mg, zidovudine 300 mg twice daily and trimethoprim-sulfamethoxazole once daily. All infants received prophylaxis with either zidovudine for 1 week or nevirapine for 6 weeks, plus trimethoprim-sulfamethoxazole from 6 weeks of age to 6 weeks after weaning. Almost all of the infants were exclusively breastfed until 6 months of age and about 73% were partially breastfed until 12 months of age. There was no statistical difference in hospitalizations or adverse events including anemia, neutropenia or deaths among infants in the two groups. Among 9 breastfed (extent not stated) infants whose mothers were taking lopinavir 400 mg with ritonavir 100 mg twice daily as part of a multi-drug treatment for HIV infection, no adverse effects were noted by investigators or reported by mothers at 1, 3 and 6 months of age. ◉ Effects on Lactation and Breastmilk Gynecomastia has been reported among men receiving highly active antiretroviral therapy. Gynecomastia is unilateral initially, but progresses to bilateral in about half of cases. No alterations in serum prolactin were noted and spontaneous resolution usually occurred within one year, even with continuation of the regimen. Some case reports and in vitro studies have suggested that protease inhibitors might cause hyperprolactinemia and galactorrhea in some male patients, although this has been disputed. The relevance of these findings to nursing mothers is not known. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Protein Binding Lopinavir is >98% protein-bound in plasma. It binds to both alpha-1-acid glycoprotein and albumin, but exhibits a greater affinity for alpha-1-acid glycoprotein. |
||
| 参考文献 | |||
| 其他信息 |
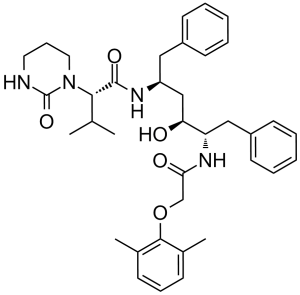
Lopinavir is a dicarboxylic acid diamide that is amphetamine is substituted on nitrogen by a (2,6-dimethylphenoxy)acetyl group and on the carbon alpha- to nitrogen by a (1S,3S)-1-hydroxy-3-{[(2S)-3-methyl-2-(2-oxotetrahydropyrimidin-1-yl)butanoyl]amino}-4-phenylbutyl group. An antiretroviral of the protease inhibitor class, it is used against HIV infections as a fixed-dose combination with another protease inhibitor, ritonavir. It has a role as an antiviral drug, a HIV protease inhibitor and an anticoronaviral agent. It is a member of amphetamines and a dicarboxylic acid diamide.
Lopinavir is an antiretroviral protease inhibitor used in combination with other antiretrovirals in the treatment of HIV-1 infection. Lopinavir is marketed and administered exclusively in combination with [ritonavir] - this combination, first marketed by Abbott under the brand name Kaletra in 2000, is necessary due to lopinavir's poor oral bioavailability and extensive biotransformation. Ritonavir is a potent inhibitor of the enzymes responsible for lopinavir metabolism, and its co-administration "boosts" lopinavir exposure and improves antiviral activity. Like many other protease inhibitors (e.g. [saquinavir], [nelfinavir]), lopinavir is a peptidomimetic molecule - it contains a hydroxyethylene scaffold that mimics the peptide linkage typically targeted by the HIV-1 protease enzyme but which itself cannot be cleaved, thus preventing the activity of the HIV-1 protease. Lopinavir was previously under investigation in combination with ritonavir for the treatment of COVID-19 caused by SARS-CoV-2. Lopinavir is a Protease Inhibitor. The mechanism of action of lopinavir is as a HIV Protease Inhibitor, and P-Glycoprotein Inhibitor, and Cytochrome P450 3A Inhibitor, and Organic Anion Transporting Polypeptide 1B1 Inhibitor. Lopinavir is an antiretroviral protease inhibitor used in combination with ritonavir in the therapy and prevention of human immunodeficiency virus (HIV) infection and the acquired immunodeficiency syndrome (AIDS). Lopinavir can cause transient and usually asymptomatic elevations in serum aminotransferase levels and, rarely, clinically apparent, acute liver injury. In HBV or HCV coinfected patients, highly active antiretroviral therapy with lopinavir may result of an exacerbation of the underlying chronic hepatitis B or C. Lopinavir is a peptidomimetic HIV protease inhibitor that retains activity against HIV protease with the Val 82 mutation. Lopinavir is less affected by binding to serum proteins than the structurally-related drug ritonavir. An HIV protease inhibitor used in a fixed-dose combination with RITONAVIR. It is also an inhibitor of CYTOCHROME P-450 CYP3A. Drug Indication The combination product lopinavir/ritonavir, marketed under the brand name Kaletra, is indicated in combination with other antiretrovirals for the treatment of HIV-1 infection in adults and pediatric patients ≥14 days old. Mechanism of Action The HIV lifecycle is comprised of 3 distinct stages: assembly, involving creation and packaging of essential viral components; budding, wherein the viral particle crosses the host cell plasma membrane and forms a lipid envelope; and maturation, wherein the viral particle alters its structure and becomes infectious. At the center of this lifecycle is the Gag polyprotein which, along with the products of its proteolysis, coordinate these stages and function as the major structural proteins of the virus. The HIV-1 protease enzyme, a dimeric aspartic protease, is the enzyme responsible for cleaving the Gag polyprotein and thus plays a critical role in many aspects of the HIV viral lifecycle. Lopinavir is an inhibitor of the HIV-1 protease enzyme. Its design is based on the "peptidomimetic" principle, wherein the molecule contains a hydroxyethylene scaffold which mimics the normal peptide linkage (cleaved by HIV protease) but which itself cannot be cleaved. By preventing HIV-1 protease activity, and thus the proteolysis of the Gag polyprotein, lopinavir results in the production of immature, non-infectious viral particles. /The researchers/ have previously shown that the HIV protease inhibitor lopinavir has selective toxicity against human papillomavirus (HPV)-positive cervical carcinoma cells via an unknown mechanism. SiHa cervical carcinoma cells were stably transfected with the proteasome sensor vector pZsProSensor-1 to confirm lopinavir inhibits the proteasome in these cells. The Panorama Xpress profiler 725 antibody array was then used to analyse specific changes in protein expression in lopinavir-treated versus control untreated SiHa cells followed by PCR and western blotting. Colorimetric growth assays of lopinavir-treated E6/E7 immortalised versus control human keratinocytes were performed. Targeted small interfering RNA gene silencing followed by growth assay comparison of lopinavir-treated/untreated SiHa cells was also used. Lopinavir induced an increase in the fluorescence of pZsProSensor-1 transfected SiHa cells, indicative of proteasomal inhibition. Ribonuclease L (RNASEL) protein was shown to be up-regulated in lopinavir-treated SiHa cells, which was confirmed by PCR and western blot. Targeted silencing of RNASEL reduced the sensitivity of SiHa cells to lopinavir. Selective toxicity against E6/E7 immortalised keratinocytes versus control cells was also seen with lopinavir and was associated with up-regulated RNASEL expression. These data are consistent with the toxicity of lopinavir against HPV-positive cervical carcinoma cells being related to its ability to block viral proteasome activation and induce an up-regulation of the antiviral protein RNASEL. This is supported by the drug's selective toxicity and up-regulation of RNASEL in E6/E7 immortalised keratinocytes combined with the increased resistance to lopinavir observed in SiHa cells following silencing of RNASEL gene expression. Lopinavir inhibits replication of HIV type 1 (HIV-1) by interfering with HIV protease. During HIV replication, HIV protease cleaves viral polypeptide products of the gag and gag-pol genes to form structural proteins of the virion core and essential viral enzymes. By interfering with the formation of these essential proteins and enzymes, lopinavir blocks maturation of the virus and causes formation of nonfunctional, immature, noninfectious virions. Lopinavir also has some in vitro activity against HIV type 2 (HIV-2). |
| 分子式 |
C37H48N4O5
|
|---|---|
| 分子量 |
628.8
|
| 精确质量 |
628.362
|
| 元素分析 |
C, 70.67; H, 7.69; N, 8.91; O, 12.72
|
| CAS号 |
192725-17-0
|
| 相关CAS号 |
(rel)-Lopinavir-d8;1322625-54-6;Lopinavir-d8;1224729-35-4
|
| PubChem CID |
92727
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
924.2±65.0 °C at 760 mmHg
|
| 熔点 |
124-127°C
|
| 闪点 |
512.7±34.3 °C
|
| 蒸汽压 |
0.0±0.3 mmHg at 25°C
|
| 折射率 |
1.577
|
| LogP |
6.26
|
| tPSA |
120
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
15
|
| 重原子数目 |
46
|
| 分子复杂度/Complexity |
940
|
| 定义原子立体中心数目 |
4
|
| SMILES |
O([H])[C@]([H])([C@]([H])(C([H])([H])C1C([H])=C([H])C([H])=C([H])C=1[H])N([H])C(C([H])([H])OC1C(C([H])([H])[H])=C([H])C([H])=C([H])C=1C([H])([H])[H])=O)C([H])([H])[C@]([H])(C([H])([H])C1C([H])=C([H])C([H])=C([H])C=1[H])N([H])C([C@]([H])(C([H])(C([H])([H])[H])C([H])([H])[H])N1C(N([H])C([H])([H])C([H])([H])C1([H])[H])=O)=O
|
| InChi Key |
KJHKTHWMRKYKJE-SUGCFTRWSA-N
|
| InChi Code |
InChI=1S/C37H48N4O5/c1-25(2)34(41-20-12-19-38-37(41)45)36(44)39-30(21-28-15-7-5-8-16-28)23-32(42)31(22-29-17-9-6-10-18-29)40-33(43)24-46-35-26(3)13-11-14-27(35)4/h5-11,13-18,25,30-32,34,42H,12,19-24H2,1-4H3,(H,38,45)(H,39,44)(H,40,43)/t30-,31-,32-,34-/m0/s1
|
| 化学名 |
(2S)-N-[(2S,4S,5S)-5-[[2-(2,6-dimethylphenoxy)acetyl]amino]-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide
|
| 别名 |
Lopinavir; ABT-378; Aluviran; Koletra; ABT 378; A-157378.0; A157378.0; A 157378.0; ABT-378; ABT378; ABT 378
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 25 mg/mL (39.76 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 250.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 配方 2 中的溶解度: ≥ 2.08 mg/mL (3.31 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: 30% PEG400+0.5% Tween80+5% propylene glycol: 30 mg/mL 配方 4 中的溶解度: 20 mg/mL (31.81 mM) in Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5903 mL | 7.9517 mL | 15.9033 mL | |
| 5 mM | 0.3181 mL | 1.5903 mL | 3.1807 mL | |
| 10 mM | 0.1590 mL | 0.7952 mL | 1.5903 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。