| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
|

| 靶点 |
5-HT7 Receptor ( IC50 = 0.495 nM ); 5-HT1A Receptor ( IC50 = 6.75 nM ); D2 Receptor ( IC50 = 1.68 nM )
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:Lurasidone 以浓度依赖性方式拮抗多巴胺刺激的 [35S]GTPγS 与人多巴胺 D2L 受体的结合,KB 值为 2.8 nM。 Lurasidone 拮抗 CHO/h5-HT7 细胞中 5-HT 刺激的 cAMP 积累,KB 值为 2.6 nM。 Lurasidone 部分刺激 [35S]GTPγS 与人 5-HT1A 受体膜制剂的结合,最大效果为 33%。 Lurasidone 剂量依赖性地增加大鼠额叶皮层和纹状体中 DOPAC/多巴胺的比率。
为了研究伊洛哌酮和卢拉西酮是否影响CYP酶的活性,用不同浓度的神经抑制剂进行了探针反应测定。Dixon的CYP特异性底物代谢图,在人类肝微粒体和超体CYP1A2、CYP2D6、CYP2C9、CYP2C19和CYP3A4中进行,在没有或有测试的神经抑制剂的情况下,表明所检查的神经抑制剂对不同的CYP酶具有抑制作用。然而,它们抑制特定CYP酶的效力是不同的。Iloperidone对CYP3A4的活性有很强的抑制作用(Ki = 0.38 肝微粒体和超体分别为0.3µM)和CYP2D6(Ki = 2.9 在肝微粒体和超体中分别为10µM)。此外,伊洛哌酮减弱了CYP2C19的活性(Ki = 6.5 肝微粒体和超体分别为32µM)和CYP1A2(Ki = 45 肝微粒体和超体分别为31µM)。Iloperidone不影响CYP2C9的活性。相比之下,鲁拉西酮适度抑制CYP1A2(Ki = 12.6 肝微粒体和超体分别为15.5µM)、CYP2C9(Ki = 18 肝微粒体和超体分别为3.5µM)、CYP2C19(Ki = 18 肝微粒体和超体分别为18.4µM)和CYP3A4(Ki = 29.4 肝微粒体和超体分别为9.1µM)。鲁拉西酮弱抑制CYP2D6的活性(Ki = 37.5 肝微粒体和超体分别为85µM)。[3] Lineweaver–Burk的酶抑制动力学图表明,在人肝微粒体和超体中,伊洛哌酮通过非竞争机制抑制CYP3A4的活性,通过竞争机制抑制CY2D6,通过混合机制抑制CYP1A2和CYP2C19的活性(插入图1、3、4、5)。另一方面,鲁拉西酮通过混合机制抑制CYP1A2、CYP2C9和CYP2C19的活性,通过竞争机制抑制CYP3A4和CYP2D6的活性(插入图1、2、3、4、5。表1[1]总结了伊洛哌酮和鲁拉西酮抑制主要人类CYP酶活性的Ki值和机制。 |
| 体内研究 (In Vivo) |
鲁拉西酮对MAP诱发的多动症的抑制作用持续8小时以上,治疗后1小时、2小时、4小时、8小时的ED50值分别为2.3mg/kg、0.87mg/kg 、 1.6 mg/kg 和 5.0 mg/kg 分别。 Lurasidone (1 mg/kg–10 mg/kg) 剂量依赖性地抑制大鼠的条件性回避反应,ED50 为 6.3 mg/kg。 Lurasidone 剂量依赖性地抑制大鼠中 TRY 诱导的前爪阵挛性癫痫发作和 p-CAMP 诱导的高热,ED50 分别为 5.6 mg/kg 和 3.0 mg/kg。 Lurasidone (0.3 mg/kg–30 mg/kg) 具有剂量依赖性,并且在 MED 为 10 mg/kg 的 Vogels 冲突试验中显着增加大鼠受到的电击次数。 Lurasidone(3 mg/kg,2 周)显着抑制嗅球切除模型大鼠的多动行为。 Lurasidone (700 mg/kg–1000 mg/kg) 以剂量依赖性方式稍微延长小鼠由六巴比妥(麻醉)引起的翻正反射丧失的持续时间。 Lurasidone(30 mg/kg,口服)可显着且剂量依赖性地逆转 MK-801 诱导的大鼠被动回避反应损伤。 Lurasidone (3 mg/kg po) 可有效逆转 Morris 水迷宫测试中 MK-801 诱导的大鼠学习障碍。 Lurasidone (3 mg/kg po) 可有效逆转 MK-801 诱导的参考记忆损伤,并在径向臂迷宫测试中适度但不显着减轻 MK-801 诱导的工作记忆损伤。 Lurasidone (10 mg/kg) 治疗会增加大鼠前额皮质中的 BDNF mRNA 总水平,并在较小程度上增加海马中的 BDNF mRNA 水平。 Lurasidone (10 mg/kg) 显着增加大鼠前额皮质中成熟 BDNF 蛋白的水平,而不影响海马提取物中神经营养蛋白(前体和成熟形式)的蛋白水平。[1]
鲁拉西酮(SM-13496)是一种新型非典型抗精神病药物,对多巴胺D2、血清素5-HT7、5-HT2A、5-HT1A受体和α2C肾上腺素受体具有高度亲和力。本研究评估了鲁拉西酮对大鼠被动回避反应的影响,以及N-甲基-d-天冬氨酸(NMDA)受体拮抗剂MK-801(地佐西平)对其的损伤,并将其与其他抗精神病药物进行了比较。在动物接受足部电击训练后1天,通过测量跨步潜伏期来检查被动回避反应。在训练课前给药时,鲁拉西酮在任何测试剂量(1-30mg/kg,口服)下都不影响被动回避反应。然而,所有其他接受检查的非典型抗精神病药物(即利培酮、奥氮平、喹硫平、氯氮平和阿立哌唑)在相对较高的剂量下显著降低了逐步潜伏期。训练前给予鲁拉西酮可显著且剂量依赖性地逆转MK-801诱导的被动回避反应损伤。在低于影响被动回避反应的剂量下,利培酮、喹硫平和氯氮平部分减轻了MK-801诱导的损伤,而氟哌啶醇、奥氮平和阿立哌唑则没有活性。此外,鲁拉西酮的训练后给药在对抗MK-801效应方面与训练前给药一样有效,这表明鲁拉西酮至少在一定程度上是通过恢复MK-801中断的记忆巩固过程起作用的。这些结果表明,鲁拉西酮在改善MK-801诱导的记忆障碍方面优于其他抗精神病药物,可能在临床上可用于治疗精神分裂症的认知障碍。[2] |
| 酶活实验 |
体外受体结合谱[1]
如表2所示,体外受体结合实验表明,鲁拉西酮对多巴胺D2和5-HT2A受体的亲和力高于其他测试的抗精神病药物。与其他药物相比,鲁拉西酮对5-HT7、5-HT1A和去甲肾上腺素α2C受体也表现出高亲和力(Ki值分别为0.495、6.75和10.8 nM)。 鲁拉西酮对去甲肾上腺素能α1和α2A受体的亲和力较低(Ki值分别为47.9和40.7 nM),亲和力可以忽略不计。.. CYP酶活性的测定[3] 为了研究伊洛哌酮和鲁拉西酮对各种CYP亚型活性的抑制作用,使用了混合的人肝微粒体和表达人CYP(超体)的杆状病毒感染昆虫细胞的微粒体。根据之前描述的方法,应用了以下探针反应:CYP1A2的咖啡因3-N-去甲基化(咖啡因200、400和800µM),CYP2C9的双氯芬酸4′-羟基化(双氯芬酸5、10、25µM);CYP2C19的哌嗪N-去甲基性(哌嗪50、100、200µM)。CYP2C9、2C19和3A4的孵育系统含有:50 mM TRIS/KCL缓冲液(pH = 7.4),NADPH生成系统(1 mM NADP、5 mM葡萄糖6-磷酸、1.7 U/ml葡萄糖6-磷酸脱氢酶、1 mM EDTA和3 mM MgCl2)。CYP1A2的孵育混合物包括:0.15 M磷酸盐缓冲液(pH = 7.4) CYP2D6:0.1 M TRIS/KCL缓冲液(pH = 7.4),NADPH生成系统(1.3 mM NADP、3.3 mM葡萄糖6-磷酸、1 U/ml葡萄糖6-磷酸脱氢酶和3.3 mM MgCl2)。加入适当浓度的人肝微粒体(每次反应0.5 mg/ml)或超体(50 pmol CYP/ml),在有或没有神经抑制剂的情况下加入不同浓度的探针底物(浓度:0.1、0.5、1、5、10µM),反应混合物的最终体积为0.5 ml。超体的孵育时间为30分钟(每次反应),肝微粒体孵育时间:30分钟(双氯芬酸4′-羟基化和丁咯洛尔1′-羟基化成),20分钟(哌嗪N-去甲基化和睾酮6β-羟基化)或50分钟(咖啡因3-N-去甲基化成)。反应停止后,如前所述,通过HPLC法结合紫外检测(或CYP2D6的荧光检测)评估肝微粒体或超体中形成的特定底物及其代谢产物的浓度。 动力学参数、Ki值和抑制机制的测定[3] 使用Michaelis-Menten方法和非线性回归分析获得了描述肝微粒体或超体中CYP特异性反应过程的动力学参数(Km、Vmax、Ki)。伊洛哌酮和鲁拉西酮对CYP酶的抑制作用如Dixon图(1/V对I)所示,显示Ki值,Lineweaver–Burk图(1/V对1/s)显示了抑制机制(竞争性抑制增加了Km值,不影响Vmax值;非竞争性抑制降低了Vmax值,但不影响Km值;混合抑制导致Km和Vmax值分别发生变化)。 |
| 动物实验 |
Methamphetamine (MAP) (1 mg/kg i.p.) is injected into each individual SD rat in a clear plastic cage one hour after the drugs or vehicle are administered. One, two, four, and eight hours prior to the MAP injection, luerazone (hydrochloride) (SM-13496 (hydrochloride)) is given as part of the persistence of effect test. Following a 10-minute MAP injection, locomotor activity is monitored for 80 minutes. The ED50 value, which inhibits MAP-induced hyperactivity by 50% of the animals tested, is determined using four or five groups of six to thirteen rats.[1]
Lurasidone hydrochloride, haloperidol, olanzapine, aripiprazole, risperidone, quetiapine hemifumarate, and clozapine were prepared. The previously reported anti-dopamine ED50 values (mg/kg, p.o.) were used to adjust the test dosage of each antipsychotic drug to a level expected to block dopamine D2 receptors in vivo, i.e., 1–30 mg/kg p.o. for Lurasidone and quetiapine; 0.3 and 1 mg/kg p.o. for haloperidol; 0.3–3 mg/kg for risperidone; 0.3–10 mg/kg for olanzapine and aripiprazole; and 0.3–30 mg/kg p.o. for clozapine (Hirose et al., 2004, Migler et al., 1993, Moore et al., 1992, Sakamoto et al., 1997). All the antipsychotic drugs were dissolved or suspended in 0.5% methylcellulose (MC) as the vehicle, and orally administered at a volume of 5 ml/kg. In the cases in which Lurasidone was injected intravenously, the drug was dissolved in 25% polyethylene glycol, and injected at 1 ml/kg into the tail vein. In this case, anti-dopaminergic doses of 0.1 and 0.3 mg/kg, which effectively antagonize methamphetamine-induced hyperactivity in rats (data not shown), were used. (+)-MK-801 hydrogen maleate was dissolved in saline and injected subcutaneously at a volume of 5 ml/kg. All the test drugs and MK-801 were prepared on the day of the experiment. All other agents were obtained from commercial sources.[2] We performed 3 sets of studies as described below. Study 1: As previously reported for clozapine and olanzapine (Ninan and Kulkarni, 1996, Rasmussen et al., 2001), some antipsychotic drugs may impair passive-avoidance learning when administered alone before the training session. Therefore, we first investigated the effects of Lurasidone and other antipsychotic drugs on the acquisition of the passive-avoidance response, when administered alone without giving MK-801. Antipsychotic drugs or the vehicle MC was administered orally 1 h before the passive-avoidance training. Ten to 15 rats per dose group were used. The data from this study were used to determine dosages of antipsychotic drugs that did not impair the passive-avoidance response. Study 2: We next examined the effect of Lurasidone on MK-801-induced deficits in the passive-avoidance response and compared the results with those of the other antipsychotic drugs. A pre-training injection of MK-801 is known to induce state-dependency in some of the context-dependent responses such as the passive avoidance in rats, which apparently impairs the retrieval of acquired response unless a pre-test injection of MK-801 is also given to rats (Harrod et al., 2001, Jackson et al., 1992, Schmidt et al., 1999). In this study, therefore, we gave both pre-training and pre-test injections of MK-801 to avoid the state-dependent influence with MK-801, according to the procedures as previously used in the passive-avoidance test (Harrod et al., 2001, Nakagawa and Iwasaki, 1996). In addition, a relatively low dose of MK-801 (0.05 mg/kg, s.c.) that reportedly does not affect motor functions and the passive-avoidance retrieval with the pre-test injection (Nakagawa and Iwasaki, 1996, Venable and Kelly, 1990) was employed. The antipsychotic drugs were administered 1 h before the training session at doses that did not impair the passive-avoidance response in Study 1. Twenty to 25 rats per dose group were used. Study 3: To investigate the interaction of Lurasidone with MK-801 specifically in the memory consolidation process of acquiring the passive-avoidance response (McGaugh, 1973, McGaugh, 2000), lurasidone was injected intravenously, 10 min after the animals received the foot-shock training and were returned to their home cages. MK-801 was given as described for Study 2. Fifteen animals per dose group were used. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Lurasidone is readily absorbed and quickly reaches maximal concentrations (Cmax) within 1-4 hours. When taken with food, there is a two-fold increase in exposure and time to maximal concentration is increased by 0.5-1.5 hours. This occurs regardless of fat or caloric content. Bioavailability = 9-19%. Urine (~9%) and feces (~80%) 6173 L 3902 mL/min Following administration of a single radiolabeled dose of lurasidone, approximately 80 and 9% of the dose is excreted in feces and urine, respectively. Lurasidone is rapidly absorbed following oral administration and reaches peak serum concentrations within about 1-3 hours. Approximately 9-19% of an administered dose is absorbed orally. Steady-state concentrations of the drug are achieved within 7 days. Metabolism / Metabolites Lurasidone is metabolized by CYP3A4 in which its major active metabolite is referred to as ID-14283 (25% of parent exposure). Its two minor metabolites are referred to as ID14326 and ID11614 which make up 3% and 1% of parent exposure respectively. Its two non-active metabolites are referred to as ID-20219 and ID-20220. Lurasidone is highly bound (99.8%) to serum proteins, including albumin and alpha1-acid glycoprotein. The drug is metabolized mainly via CYP3A4. The major biotransformation pathways are oxidative N-dealkylation, hydroxylation of the norbornane ring, and S-oxidation. Lurasidone is metabolized into 2 active metabolites (ID-14283 and ID-14326) and 2 major inactive metabolites (ID-20219 and ID-20220). Biological Half-Life 40 mg dose= 18 hours 120 mg - 160 mg dose = 29-37 hours |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Lurasidone is more than 99% bound to plasma proteins, so it is unlikely that the drug would be excreted into milk in sufficient amounts to affect a breastfed infant. Data from one mother-infant pair appears to support the poor excretion into milk and lack of effect on the breastfed infant. Until more data are available, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants A woman with depressive type schizoaffective disorder was taking lurasidone 40 mg at night and desvenlafaxine 50 mg daily after giving birth. She exclusively breastfed her infant. The infant’s growth and development was good during a follow-up period of 39 days. Patients enlisted in the National Pregnancy Registry for Atypical Antipsychotics who were taking a second-generation antipsychotic drug while breastfeeding (n = 576) were compared to control breastfeeding patients who were not treated with a second-generation antipsychotic (n = 818). Of the patients who were taking a second-generation antipsychotic drug, 60.4% were on more than one psychotropic. A review of the pediatric medical records, no adverse effects were noted among infants exposed or not exposed to second-generation antipsychotic monotherapy or to polytherapy. The number of women taking lurasidone was not reported. ◉ Effects on Lactation and Breastmilk Increases in serum prolactin with lurasidone are generally infrequent, small and less than risperidone. A woman with elevated serum prolactin, breast tenderness and galactorrhea while taking risperidone improved when lurasidone was substituted for risperidone and these side effects subsided completely when the lurasidone dose was increased from 20 mg to 40 mg daily. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Patients enlisted in the National Pregnancy Registry for Atypical Antipsychotics who were taking a second-generation antipsychotic drug while breastfeeding (n = 576) were compared to control breastfeeding patients who had primarily diagnoses of major depressive disorder and anxiety disorders, most often treated with SSRI or SNRI antidepressants, but not with a second-generation antipsychotic (n = 818). Among women on a second-generation antipsychotic, 60.4% were on more than one psychotropic compared with 24.4% among women in the control group. Of the women on a second-generation antipsychotic, 59.3% reported “ever breastfeeding” compared to 88.2% of women in the control group. At 3 months postpartum, 23% of women on a second-generation antipsychotic were exclusively breastfeeding compared to 47% of women in the control group. The number of women taking lurasidone was not reported. A 14-year-old girl with hallucinatory schizophrenia was treated inadequately with aripiprazole, then paliperidone. As she was transitioned from paliperidone to lurasidone at age 16 years, her serum prolactin increased to 4240 mIU/L (normal range 60-400 mIU/L). As the lurasidone dose was titrated to a maximum of 111 mg daily, prolactin levels continued to increase and the patient experienced breast fullness and galactorrhea. Six of 7 serum prolactin measurements were in the range of 4240 to 6140 mIU/L. Once lurasidone was discontinued, her serum prolactin normalized. In an Italian study of treatment of schizophrenic patients with lurasidone, 2.4% of patients developed hyperprolactinemia and galactorrhea. Toxicity Summary IDENTIFICATION AND USE: Lurasidone is indicated for the treatment of patients with schizophrenia, as monotherapy for the treatment of patients with major depressive episodes associated with bipolar I disorder (bipolar depression), and as adjunctive therapy with either lithium or valproate for the treatment of patients with major depressive episodes associated with bipolar I disorder (bipolar depression). HUMAN EXPOSURE AND TOXICITY: An increased incidence of adverse cerebrovascular events (cerebrovascular accidents and transient ischemic attacks), including fatalities, has been observed in geriatric patients with dementia-related psychosis treated with certain atypical antipsychotic agents (aripiprazole, olanzapine, risperidone) in placebo-controlled studies. The manufacturer states that lurasidone is not approved for the treatment of patients with dementia-related psychosis. Neuroleptic malignant syndrome (NMS), a potentially fatal syndrome requiring immediate discontinuance of the drug and intensive symptomatic treatment, has been reported in patients receiving antipsychotic agents, including lurasidone. Rash and pruritus have been reported frequently and angioedema has been reported rarely in patients receiving lurasidone. Adverse effects occurring in 5% or more of patients receiving lurasidone for schizophrenia and at a frequency at least twice that reported with placebo include somnolence (including hypersomnia, hypersomnolence, and sedation), akathisia, nausea, parkinsonism, and agitation. Akathisia and somnolence appear to be dose-related adverse effects.The effect of lurasidone on labor and delivery is unknown. It is not known whether lurasidone and/or its metabolites are distributed into milk in humans. In geriatric patients (65-85 years of age) with psychosis, serum lurasidone concentrations were similar to those observed in younger adults. Geriatric patients with dementia-related psychosis treated with lurasidone are at an increased risk of death compared with those treated with placebo. Safety and effectiveness of lurasidone in pediatric and adolescent patients have not been established. ANIMAL STUDIES: Lurasidone increased the incidence of mammary gland carcinomas in females rats orally dosed at 12 and 36 mg/kg/day: the lowest dose; 3 mg/kg/day is the no-effect dose which produced plasma levels (AUC) 0.4-times those in humans receiving the MRHD. No increases in tumors were seen in male rats up to the highest dose tested, which produced plasma levels (AUC) 6-times those in humans receiving the MRHD. Lurasidone is distributed into milk in rats. Estrus cycle irregularities were seen in rats orally administered lurasidone at 1.5, 15 and 150 mg/kg/day for 15 consecutive days prior to mating, during the mating period, and through day 7 of gestation. The no-effect dose is 0.1 mg/kg which is approximately 0.006-times the MRHD of 160 mg/day based on body surface area. Fertility was reduced only at the highest dose, which was reversible after a 14-day drug-free period. The no-effect dose for reduced fertility was 15 mg/kg, which is approximately equal to the MRHD based on body surface area. Lurasidone had no effect on fertility in male rats treated orally with lurasidone for 64 consecutive days prior to mating and during the mating period at doses up to 150 mg/kg/day (9-times the MRHD based on mg/m sq body surface area). The drug did not cause mutation or chromosomal aberration when tested in vitro and in vivo. It was negative in the Ames gene mutation test, the Chinese Hamster Lung (CHL) cells, and in the in vivo mouse bone marrow micronucleus test up to 2000 mg/kg (61 times the MRHD of 160 mg/day based on mg/ sq m body surface area). Interactions Lurasidone is not a substrate for CYP1A2 in vitro; therefore, smoking should not alter the pharmacokinetics of the drug. Concomitant administration of rifampin (600 mg daily for 8 days), a strong CYP3A4 inducer, and lurasidone (single 40-mg dose) decreased peak serum lurasidone concentrations and AUCs by approximately 86 and 80%, respectively. Rifampin should not be concurrently administered with lurasidone. Concomitant administration of lurasidone (40 mg daily at steady state) with an oral contraceptive containing ethinyl estradiol and norgestimate resulted in equivalent peak plasma concentrations and AUCs of ethinyl estradiol and norgestimate relative to oral contraceptive administration alone. Sex hormone binding globulin concentrations also were not substantially affected by concurrent administration of the drugs. Oral contraceptive dosage adjustment is not required in patients receiving lurasidone concurrently. Concomitant administration of lurasidone (120 mg daily at steady state) with a single 5-mg dose of midazolam, a CYP3A4 substrate, increased peak plasma concentrations and AUCs of midazolam by approximately 21 and 44%, respectively. Midazolam dosage adjustment is not required in patients receiving lurasidone concurrently. For more Interactions (Complete) data for Lurasidone (11 total), please visit the HSDB record page. Hepatotoxicity Liver test abnormalities occur in 1% to 3% of patients on long term therapy with lurasidone, but similar rates have been reported with placebo therapy and with comparator agents. The ALT elevations are usually mild, transient and often resolve even without dose modification or drug discontinuation. There have been no published reports of clinically apparent liver injury with symptoms or jaundice attributed to lurasidone therapy. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Lurasidone is more than 99% bound to plasma proteins, so it is unlikely that the drug would be excreted into milk in sufficient amounts to affect a breastfed infant. Data from one mother-infant pair appears to support the poor excretion into milk and lack of effect on the breastfed infant. Until more data are available, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants A woman with depressive type schizoaffective disorder was taking lurasidone 40 mg at night and desvenlafaxine 50 mg daily after giving birth. She exclusively breastfed her infant. The infant’s growth and development was good during a follow-up period of 39 days. Patients enlisted in the National Pregnancy Registry for Atypical Antipsychotics who were taking a second-generation antipsychotic drug while breastfeeding (n = 576) were compared to control breastfeeding patients who were not treated with a second-generation antipsychotic (n = 818). Of the patients who were taking a second-generation antipsychotic drug, 60.4% were on more than one psychotropic. A review of the pediatric medical records, no adverse effects were noted among infants exposed or not exposed to second-generation antipsychotic monotherapy or to polytherapy. The number of women taking lurasidone was not reported. ◉ Effects on Lactation and Breastmilk Increases in serum prolactin with lurasidone are generally infrequent, small and less than risperidone. A woman with elevated serum prolactin, breast tenderness and galactorrhea while taking risperidone improved when lurasidone was substituted for risperidone and these side effects subsided completely when the lurasidone dose was increased from 20 mg to 40 mg daily. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Patients enlisted in the National Pregnancy Registry for Atypical Antipsychotics who were taking a second-generation antipsychotic drug while breastfeeding (n = 576) were compared to control breastfeeding patients who had primarily diagnoses of major depressive disorder and anxiety disorders, most often treated with SSRI or SNRI antidepressants, but not with a second-generation antipsychotic (n = 818). Among women on a second-generation antipsychotic, 60.4% were on more than one psychotropic compared with 24.4% among women in the control group. Of the women on a second-generation antipsychotic, 59.3% reported “ever breastfeeding” compared to 88.2% of women in the control group. At 3 months postpartum, 23% of women on a second-generation antipsychotic were exclusively breastfeeding compared to 47% of women in the control group. The number of women taking lurasidone was not reported. A 14-year-old girl with hallucinatory schizophrenia was treated inadequately with aripiprazole, then paliperidone. As she was transitioned from paliperidone to lurasidone at age 16 years, her serum prolactin increased to 4240 mIU/L (normal range 60-400 mIU/L). As the lurasidone dose was titrated to a maximum of 111 mg daily, prolactin levels continued to increase and the patient experienced breast fullness and galactorrhea. Six of 7 serum prolactin measurements were in the range of 4240 to 6140 mIU/L. Once lurasidone was discontinued, her serum prolactin normalized. In an Italian study of treatment of schizophrenic patients with lurasidone, 2.4% of patients developed hyperprolactinemia and galactorrhea. Drugs and Lactation Database (LactMed) ◈ What is lurasidone? Lurasidone is an antipsychotic medication that has been used to treat schizophrenia and bipolar depression. It is sold under the brand name Latuda®.Sometimes when people find out they are pregnant, they think about changing how they take their medication, or stopping their medication altogether. However, it is important to talk with your healthcare providers before making any changes to how you take this medication. Your healthcare providers can talk with you about the benefits of treating your condition and the risks of untreated illness during pregnancy. ◈ I take lurasidone. Can it make it harder for me to get pregnant? Studies have not been done in humans to see if lurasidone can make it harder to get pregnant. ◈ Does taking lurasidone increase the chance for miscarriage? Miscarriage can occur in any pregnancy. Studies have not been done to see if lurasidone can increase the chance for miscarriage. ◈ Does taking lurasidone increase the chance of birth defects?* Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Information on the use of lurasidone in pregnancy is limited. Animal studies in rats and rabbits have not shown an increased chance of birth defects. In a case report of a person taking lurasidone throughout pregnancy, the baby was born healthy and without birth defects. A study looking at 134 people who used lurasidone in pregnancy found no specific patterns of birth defects. ◈ Does taking lurasidone in pregnancy increase the chance of other pregnancy-related problems? Studies have not been done to see if lurasidone use in pregnancy increases the chance for pregnancy-related problems such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth). ◈ I need to take lurasidone throughout my entire pregnancy. Will it cause symptoms in my baby after birth? Product labels written by the U.S. Food and Drug Administration (FDA) note a chance for symptoms in newborns exposed to antipsychotic drugs in the third trimester of pregnancy. Symptoms may include uncontrolled muscle movements, changes in muscle tone, being too sleepy, trouble with breathing, and/or trouble with feeding. Not all babies who are exposed to antipsychotic drugs during pregnancy will have these symptoms. These symptoms can be temporary and can go away on their own. Treatment of symptoms can be started, if needed.These symptoms have not been reported with exposure to lurasidone during pregnancy. The available information on the use of lurasidone in pregnancy is so limited that it is hard to know if these symptoms might happen. Let your healthcare providers know before delivery if you are taking lurasidone. If needed, babies can be monitored for symptoms. ◈ Does taking lurasidone in pregnancy affect future behavior or learning for the child? Studies have not been done to see if lurasidone use in pregnancy can cause behavior or learning issues for the child. ◈ Breastfeeding while taking lurasidone: Information on the use of lurasidone while breastfeeding is limited. There is a report of one person who was taking lurasidone while breastfeeding. No negative effects were reported in the nursing child. The benefit of using lurasidone may outweigh possible risks. Your healthcare providers can talk with you about using lurasidone and what treatment is best for you. Be sure to talk to your healthcare provider about all your breastfeeding questions. ◈ If a male takes lurasidone, could it affect fertility (ability to get partner pregnant) or increase the chance of birth defects? Studies have not been done in humans to see if lurasidone could affect fertility or increase the chance of birth defects above the background risk. In general, exposures that fathers or sperm donors have are unlikely to increase the risks to a pregnancy. For more information, please see the MotherToBaby fact sheet Paternal Exposures at https://mothertobaby.org/fact-sheets/paternal-exposures-pregnancy/. |
| 参考文献 | |
| 其他信息 |
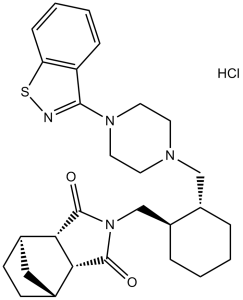
Lurasidone hydrochloride is a hydrochloride obtained by reaction of lurasidone with one equivalent of hydrochloric acid. An atypical antipsychotic agent used for the treatment of schizophrenia. It has a role as a dopaminergic antagonist, a serotonergic antagonist, an adrenergic antagonist and a second generation antipsychotic. It contains a lurasidone(1+).
A thiazole derivative and atypical ANTIPSYCHOTIC AGENT that functions as a DOPAMINE D2 RECEPTOR ANTAGONIST; SEROTONIN 5-HT2 RECEPTOR ANTAGONIST, serotonin 5-HT7 receptor antagonist, and antagonist of the adrenergic α2A and α2C receptors, as well as a partial SEROTONIN 5-HT1A RECEPTOR AGONIST. It is used in the treatment of SCHIZOPHRENIA and BIPOLAR DISORDER. See also: Lurasidone (has active moiety). Drug Indication Treatment of schizophrenia in adults aged 18 years and over. Treatment of schizophrenia. Lurasidone [(3aR,4S,7R,7aS)-2-[(1R,2R)-2-[4-(1,2-benzisothiazol-3-yl)piperazin-1-ylmethyl]cyclohexylmethyl]hexahydro-4,7-methano-2H-isoindole-1,3-dione hydrochloride; SM-13496] is an azapirone derivative and a novel antipsychotic candidate. The objective of the current studies was to investigate the in vitro and in vivo pharmacological properties of lurasidone. Receptor binding affinities of lurasidone and several antipsychotic drugs were tested under comparable assay conditions using cloned human receptors or membrane fractions prepared from animal tissue. Lurasidone was found to have potent binding affinity for dopamine D(2), 5-hydroxytryptamine 2A (5-HT(2A)), 5-HT(7), 5-HT(1A), and noradrenaline alpha(2C) receptors. Affinity for noradrenaline alpha(1), alpha(2A), and 5-HT(2C) receptors was weak, whereas affinity for histamine H(1) and muscarinic acetylcholine receptors was negligible. In vitro functional assays demonstrated that lurasidone acts as an antagonist at D(2) and 5-HT(7) receptors and as a partial agonist at the 5-HT(1A) receptor subtype. Lurasidone showed potent effects predictive of antipsychotic activity, such as inhibition of methamphetamine-induced hyperactivity and apomorphine-induced stereotyped behavior in rats, similar to other antipsychotics. Furthermore, lurasidone had only weak extrapyramidal effects in rodent models. In animal models of anxiety disorders and depression, treatment with lurasidone was associated with significant improvement. Lurasidone showed a preferential effect on the frontal cortex (versus striatum) in increasing dopamine turnover. Anti-alpha(1)-noradrenergic, anticholinergic, and central nervous system (CNS) depressant actions of lurasidone were also very weak. These results demonstrate that lurasidone possesses antipsychotic activity and antidepressant- or anxiolytic-like effects with potentially reduced liability for extrapyramidal and CNS depressant side effects. [1] Background The present study aimed at examining the inhibitory effect of two atypical neuroleptics iloperidone and lurasidone on the main human cytochrome P450 (CYP) enzymes in pooled human liver microsomes and cDNA-expressed CYP enzymes (supersomes). Methods The activity of these enzymes was determined by the following CYP-specific reactions: caffeine 3-N-demethylation/CYP1A2, diclofenac 4′-hydroxylation/CYP2C9, perazine N-demethylation/CYP2C19, bufuralol 1′-hydroxylation/CYP2D6 and testosterone 6β-hydroxylation/CYP3A4, respectively, using HPLC. Results Iloperidone inhibited the activity of CYP3A4 via a noncompetitive mechanism (Ki = 0.38 and 0.3 µM in liver microsomes and supersomes, respectively) and CYP2D6 via a competitive mechanism (Ki = 2.9 and 10 µM in microsomes and supersomes). Moreover, iloperidone attenuated the activity of CYP1A2 (Ki = 45 and 31 µM in microsomes and supersomes) and CYP2C19 via a mixed mechanism (Ki = 6.5 and 32 µM in microsomes and supersomes) but did not affect CYP2C9. Lurasidone moderately inhibited CYP1A2 (Ki = 12.6 and 15.5 µM in microsomes and supersomes), CYP2C9 (Ki = 18 and 3.5 µM in microsomes and supersomes) and CYP2C19 via a mixed mechanism (Ki = 18 and 18.4 µM in microsomes and supersomes), and CYP3A4 via a competitive mechanism (Ki = 29.4 and 9.1 µM in microsomes and supersomes). Moreover, lurasidone competitively, though weakly diminished the CYP2D6 activity (Ki = 37.5 and 85 µM in microsomes and supersomes). Conclusion The examined neuroleptics showed inhibitory effects on different CYP enzymes. The obtained results indicate that metabolic/pharmacokinetic interactions with iloperidone (involving mainly CYP3A4 and CYP2D6) and possibly with lurasidone (involving CYP1A2, CYP2C9 or CYP2C19) may occur during combined therapy.[3] |
| 分子式 |
C28H37CLN4O2S
|
|---|---|
| 分子量 |
529.14
|
| 精确质量 |
528.23
|
| 元素分析 |
C, 63.56; H, 7.05; Cl, 6.70; N, 10.59; O, 6.05; S, 6.06
|
| CAS号 |
367514-88-3
|
| 相关CAS号 |
Lurasidone; 367514-87-2; Lurasidone metabolite 14326 hydrochloride; Lurasidone-d8 hydrochloride; Lurasidone Metabolite 14283-d8; 2070009-43-5; Lurasidone metabolite 14326; 186204-33-1; Lurasidone Metabolite 14326-d8
|
| PubChem CID |
11237860
|
| 外观&性状 |
White to off-white solid powder
|
| 熔点 |
198-205°C
|
| 闪点 |
9℃
|
| LogP |
4.196
|
| tPSA |
84.99
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
36
|
| 分子复杂度/Complexity |
804
|
| 定义原子立体中心数目 |
6
|
| SMILES |
O=C([C@H]([C@H]1CC[C@@H]2C1)[C@H]2C3=O)N3C[C@@H]4CCCC[C@H]4CN(CC5)CCN5C6=NSC7=CC=CC=C76.Cl
|
| InChi Key |
NEKCRUIRPWNMLK-SCIYSFAVSA-N
|
| InChi Code |
InChI=1S/C28H36N4O2S.ClH/c33-27-24-18-9-10-19(15-18)25(24)28(34)32(27)17-21-6-2-1-5-20(21)16-30-11-13-31(14-12-30)26-22-7-3-4-8-23(22)35-29-26;/h3-4,7-8,18-21,24-25H,1-2,5-6,9-17H2;1H/t18-,19+,20-,21-,24+,25-;/m0./s1
|
| 化学名 |
(1S,2R,6S,7R)-4-[[(1R,2R)-2-[[4-(1,2-benzothiazol-3-yl)piperazin-1-yl]methyl]cyclohexyl]methyl]-4-azatricyclo[5.2.1.02,6]decane-3,5-dione;hydrochloride
|
| 别名 |
SM13496; Lurasidone HCl; SM-13496; 367514-88-3; Lurasidone hydrochloride;; UNII-O0P4I5851I; CHEBI:70732; O0P4I5851I; lurasidone monohydrochloride; HCl, Lurasidone; SM 13496; trade name Latuda
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 0.67 mg/mL (1.27 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 6.7 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 0.67 mg/mL (1.27 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 6.7mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8899 mL | 9.4493 mL | 18.8986 mL | |
| 5 mM | 0.3780 mL | 1.8899 mL | 3.7797 mL | |
| 10 mM | 0.1890 mL | 0.9449 mL | 1.8899 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05213143 | Active Recruiting |
Drug: Lurasidone | Schizophrenia | Sumitomo Pharma (Suzhou) Co., Ltd. | December 30, 2021 | Phase 4 |
| NCT03395392 | Active Recruiting |
Drug: NRX-101 Drug: Lurasidone HCl |
Bipolar Depression Suicidal Ideation and Behavior |
Second Affiliated Hospital of Guangzhou Medical University |
May 12, 2022 | Phase 2 Phase 3 |
| NCT03396068 | Active Recruiting |
Drug: Lurasidone HCl Drug: NRX-101 |
Bipolar Depression Suicidal Ideation |
NeuroRx, Inc. | December 1, 2019 | Phase 3 |
| NCT02731612 | Recruiting | Drug: lurasidone Drug: Placebo |
Bipolar Disorder | Nazlin Walji | May 8, 2017 | Phase 3 |
| NCT05351736 | Recruiting | Drug: Lurasidone | Schizophrenia | Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico |
January 26, 2022 | Phase 4 |
|
|---|
|
|