| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
Microbial Metabolite; Endogenous Metabolite
|
|---|---|
| 体外研究 (In Vitro) |
人 YTH 结构域家族 2 (YTHDF2) 蛋白特异性识别 N6-甲基腺苷 (m6A) 并用它来控制 mRNA 的降解。所有真核生物信使 RNA 都含有 N6-甲基腺苷 (m6A),这是一种广泛存在的内部改变。 M6A 甲基转移酶(例如 MT-A70)在转录后将 m6A 安装在共有序列 (m6A)C (30%) 内的 G(m6A)C(70%) 或 A 上。含有 N6-甲基腺苷 (m6A) 的 RNA 在流通级分中显着减少,而在 YTHDF 结合级分中大大富集 [1]。最常见的内部 RNA 修饰 N6-甲基腺苷 (m6A) 涉及许多生物学功能,包括控制胚胎干细胞的自我更新和分化。类甲基转移酶 3 (METTL3) 和类甲基转移酶 14 (METTL14) 的催化亚基构成了称为 N6-甲基腺苷 (m6A) 的大型蛋白质复合物的一部分 [2]。
|
| 体内研究 (In Vivo) |
研究表明,由肝细胞分泌的细胞因子CCL3通过virlike m6A methyltransferase associated (VIRMA)在ICC细胞中调节m6A修饰,从而促进肿瘤转移。此外,免疫组织化学分析显示,VIRMA与ICC患者的不良预后相关。最后,我们在体外和体内都证实了CCL3可以激活VIRMA及其关键下游靶点SIRT1,从而促进ICC的肿瘤转移。
结论:
总之,研究结果增强了我们对肝细胞与ICC细胞相互作用的理解,揭示了CCL3/VIRMA/SIRT1通路通过m6A介导的ICC转移调控的分子机制。这些研究强调了ICC的诊断、治疗和预后的潜在靶点。[4]
|
| 酶活实验 |
EMSA (electrophotic Mobility Shift Assay / Gel Shift Assay) [1]
该RNA探针由先前报道的方法合成,序列为5 ' - augggccguucaucugcuaaaaggxcugcuuuuggcuugu -3 ' (X = a或m6A)。合成完成后,RNA探针在2µL RNA探针(1µM)、5µL 5×T4 PNK缓冲液a (Fermentas)、1µL T4 PNK (Fermentas)、1µL32P-ATP和41µL无rnase水(终RNA浓度40 nM)的反应混合物中进行标记,37℃下反应1小时。然后用生物凝胶P30在Tris缓冲液(BioRad 732-6250)中使用无rnase微自旋柱纯化混合物,以去除热ATP和其他小分子。洗脱液中加入2.5µL 20 × SSC缓冲液。将混合物加热至65℃10 min使RNA探针变性,然后缓慢冷却至室温。GST-YTHDF1-3在结合缓冲液(10 mM HEPES, pH 8.0, 50 mM KCl, 1mM EDTA, 0.05% trion - x -100, 5%甘油,10µg/mL Salmon DNA, 1mM DTT和40 U/mL RNasin)中稀释至200 nmol, 1µM, 5µM, 20µM和100µM(或其他指定浓度)的浓度系列。上孔前,加入1µL RNA探针(终浓度为4 nM)和1µL蛋白(终浓度为20 nM、100 nM、500 nM、2µM或10µM),在冰上孵育30 min。将整个10µL RNA-蛋白混合物上胶(Novex 4~20% TBE凝胶),在4°C、90 V下运行90 min。利用存储荧光粉屏(K-Screen;富士胶片)和Bio-Rad分子成像仪FX与Quantity One软件(Bio-Rad)相结合。通过Origin 8软件的非线性曲线拟合(Function Hyperbl)计算Kd(解离常数),y = P1×x/(P2+x),其中y为[RNA-蛋白]/[游离RNA]+[RNA-蛋白]的比值,x为蛋白浓度,P2为Kd。 体外Pull down[1] 0.8µg mRNA(节省0.2µg从相同的样本作为输入)和YTHDF1-3 C-YTHDF2 500海里)(最终浓度被稀释到200µL IPP缓冲区(150毫米氯化钠0.1% NP-40 10毫米三羟甲基氨基甲烷、液pH值7.4,40 U /毫升核糖核酸酶抑制剂,0.5毫米德勤),和旋转的解决方案是混合在4°C 2 h。YTHDF1-3, 10µL GST-affinity磁珠(皮尔斯)被用于每个样本后洗四次与200µL为每个洗IPP缓冲区。对于C-YTHDF2,用20µL Dynabeads®His-Tag Isolation & Pulldown beads (Invitrogen)洗涤四次,每次洗涤200µL IPP缓冲液。然后将微球重新悬浮在50µL IPP缓冲液中。将蛋白质- rna混合物与GST或His6珠结合,在4°C下旋转2小时。水相收集,乙醇沉淀法回收,溶解于15µL水中,保存为流道。每次用300µL IPP缓冲液洗涤四次。在珠中加入0.4 mL三唑试剂,并按照制造商的说明进一步纯化。纯化后的部分溶解于15µL水中,保存为ythdf结合。采用LC-MS/MS测量输入、流量和ythdf结合的每个样品中m6A的水平。 LC/MS [1]< br > 200 - 300 ng消化mRNA的核酸酶P1 (2 U)在25µl氯化钠的缓冲区包含25毫米,2.5毫米的优化选取37°C 2 h,紧随其后的是添加NH4HCO3 (1 M, 3µl)和碱性磷酸酶(0.5 U)。额外的孵化后37°C 2 h,样品稀释至50µl和过滤(0.22µM孔隙大小,直径约4毫米,微孔),和5µl的解决方案是注入质/女士。在C18柱上采用反相超高效液相色谱分离核苷,在线质谱检测采用Agilent 6410 QQQ三重四极杆LC质谱仪,电喷雾电离模式。采用282 ~ 150 (m6A)和268 ~ 136 (A)的核苷-碱离子质量转移进行定量。与在同一批样品上运行的纯核苷标准品获得的标准曲线进行定量。根据校准后的浓度计算出m6A / A的比值。 |
| 细胞实验 |
m6A profiling [1]
用TRIZOL试剂从HeLa细胞中分离总RNA。使用FastTrack MAG Maxi mRNA分离试剂盒从总RNA中进一步富集Poly(A)+ RNA。特别地,对所有样品进行了额外的DNase I消化步骤,以避免DNA污染。RNA片段、m6A-seq和文库制备按照Dominissini等人先前制定的方案进行。试验分2个生物重复进行(扩展数据表1)。 N(6)-甲基腺苷(m(6)A)是最丰富的内部RNA修饰,在多种生物过程中起作用,包括调节胚胎干细胞的自我更新和分化。到目前为止,检测转录组中m(6)A的方法依赖于m(6)A抗体的可用性和质量,并且通常与高假阳性率相关。在这里,基于我们对m(6)A干扰A- t /U配对的观察,我们报告了一种基于微阵列的技术来绘制小鼠胚胎干细胞中的m(6)A位点。我们从66个PolyA rna中鉴定出72个具有高m(6)A水平的无偏位点。生物信息学分析表明,鉴定的位点富含发育调节因子,并可能在某些情况下调节microRNA/mRNA的相互作用。总的来说,我们已经开发了基于微阵列的技术来捕获哺乳动物转录组中高度富集的m(6)A位点。该方法为某些应用提供了识别m(6)A位点的替代方法。[2] |
| 动物实验 |
Female Balb/c nude mice (Balb/c-nu, weighing ~ 15–20 g), were used. To generate a subcutaneous xenograft tumor model, we injected 5 × 106 transfected HuCCT1 cells, which were suspended in 0.2 ml of PBS, into the flank of nude mice (five mice per group). On the other hand, an orthotopic xenograft tumor model was established by inoculating 3 × 106 transfected HuCCT1 cells in 50 μl of PBS into the liver of nude mice (five mice per group). For the treated groups, HuCCT1 cells were treated with CCL3 (100 ng/ml) for 6 h before inoculation. For subcutaneous injection, the intratumoral multiple-point injection of CCL3 (20 mg/kg) diluted in 50 μl PBS was performed every 5 days. The control groups were treated with PBS. Subcutaneous tumor size was measured twice a week. After in vivo fluorescence imaging, all the study mice were sacrificed with the in vivo imaging system (IVIS) spectrum after four weeks. The mice tumors and organs were dissected, photographed, weighed, and stained. [4]
Background: Intrahepatic cholangiocarcinoma (ICC) is a malignant disease characterized by onset occult, rapid progression, high relapse rate, and high mortality. However, data on how the tumor microenvironment (TME) regulates ICC metastasis at the transcriptomic level remains unclear. This study aimed to explore the mechanisms and interactions between hepatocytes and ICC cells. Methods: We analyzed the interplay between ICC and liver microenvironment through cytokine antibody array analysis. Then we investigated the role of N6-methyladenosine (m6A) modification and the downstream target in vitro, in vivo experiments, and in clinical specimens. [4] |
| 参考文献 | |
| 其他信息 |
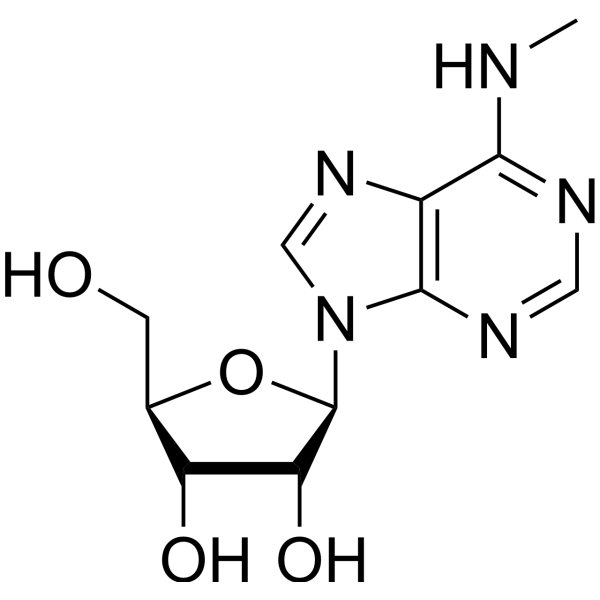
N(6)-methyladenosine is a methyladenosine compound with one methyl group attached to N(6) of the adenine nucleobase.
N6-methyladenosine (m6A), as a dynamic posttranscriptional RNA modification, recently gave rise to the field of viral epitranscriptomics. The interaction between virus and host is affected by m6A. Multiple m6A-modified viral RNAs have been observed. The epitranscriptome of m6A in host cells are altered after viral infection. The expression of viral genes, the replication of virus and the generation of progeny virions are influenced by m6A modifications in viral RNAs during virus infection. Meanwhile, the decorations of m6A in host mRNAs can make viral infections more likely to happen or can enhance the resistance of host to virus infection. However, the mechanism of m6A regulation in viral infection and host immune response has not been thoroughly elucidated to date. With the development of sequencing-based biotechnologies, transcriptome-wide mapping of m6A in viruses has been achieved, laying the foundation for expanding its functions and corresponding mechanisms. In this report, we summarize the positive and negative effects of m6A in distinct viral infection. Given the increasingly important roles of m6A in diverse viruses, m6A represents a novel potential target for antiviral therapy. [3] In our study, we only employed the m6A dot blot assay to quantify the m6A levels of ICC, and showed that SIRT1 is a downstream target of VIRMA. Thus, there is a need for further studies which would take the following variables into account: (1) examine the m6A levels of ICC using a colorimetric strategy or liquid chromatography-mass spectrometry (LC–MS), (2) detect whether VIRMA functions independently of its m6A catalytic activity in cancer progression, and (3) develop a peptide inhibitor to target VIRMA domain and explore whether it may be beneficial in the treatment of ICC. In conclusion, our study highlights the critical role of VIRMA-mediated m6A modification in ICC progression and metastasis. Mechanistically, we demonstrated that CCL3 is secreted by hepatocytes and may promote metastasis of ICC cells by regulating m6A methylation. The regulation of m6A methylation is mediated by VIRMA, which epigenetically promotes SIRT1 expression through an m6A methylation-dependent mechanism. Our results suggested that the interaction between hepatocytes and ICC cells might offer a possible interventional target for ICC. Besides, the m6A modification on tumor metastasis will contribute to further studies that would explore molecular mechanisms and identify efficient treatment strategies against ICC. [4] |
| 分子式 |
C11H15N5O4
|
|---|---|
| 分子量 |
281.27
|
| 精确质量 |
281.112
|
| 元素分析 |
C, 46.97; H, 5.38; N, 24.90; O, 22.75
|
| CAS号 |
1867-73-8
|
| 相关CAS号 |
N6-Methyladenosine-d3;139896-43-8
|
| PubChem CID |
102175
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.85g/cm3
|
| 沸点 |
649.1ºC at 760mmHg
|
| 闪点 |
346.3ºC
|
| 蒸汽压 |
9.86E-18mmHg at 25°C
|
| 折射率 |
1.814
|
| LogP |
-0.4
|
| tPSA |
125.55
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
20
|
| 分子复杂度/Complexity |
349
|
| 定义原子立体中心数目 |
4
|
| SMILES |
CNC1=C2C(=NC=N1)N(C=N2)[C@H]3[C@@H]([C@@H]([C@H](O3)CO)O)O
|
| InChi Key |
VQAYFKKCNSOZKM-IOSLPCCCSA-N
|
| InChi Code |
InChI=1S/C11H15N5O4/c1-12-9-6-10(14-3-13-9)16(4-15-6)11-8(19)7(18)5(2-17)20-11/h3-5,7-8,11,17-19H,2H2,1H3,(H,12,13,14)/t5-,7-,8-,11-/m1/s1
|
| 化学名 |
(2R,3S,4R,5R)-2-(hydroxymethyl)-5-[6-(methylamino)purin-9-yl]oxolane-3,4-diol
|
| 别名 |
6-Methylaminopurinosine; N6-Methyladenosine; m6A; 1867-73-8; N-Methyladenosine; 6-Methyladenosine; Adenosine, N-methyl-; N(6)-Methyladenosine; 6-Methylaminopurinosine; 6-Methylaminopurine riboside;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 31 mg/mL (~110.21 mM)
H2O : ~5.56 mg/mL (~19.77 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (7.40 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (7.40 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (7.40 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.5553 mL | 17.7765 mL | 35.5530 mL | |
| 5 mM | 0.7111 mL | 3.5553 mL | 7.1106 mL | |
| 10 mM | 0.3555 mL | 1.7777 mL | 3.5553 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。