| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
Topoisomerase IV; Topoisomerase II
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:马波沙星是一种专为兽医用途开发的氟喹诺酮抗菌剂。 Marbofloxacin 对多种需氧革兰氏阴性菌和一些革兰氏阳性菌以及支原体表现出高杀菌活性。作为第三代氟喹诺酮类药物,马波沙星还主要靶向复制和转录酶,例如 DNA 旋转酶和拓扑异构酶 IV,这两种酶对于细菌的活力都至关重要。在猪肺炎支原体 116 野生型菌株和马波沙星以治疗剂量体内治疗 4 天后分离的克隆中,马波沙星在指数期具有支原体作用,但在滞后期不具有支原体作用。马波沙星以剂量依赖性方式显着杀死利什曼原虫前鞭毛体和细胞内无鞭毛体,比葡甲胺锑酸盐和葡萄糖酸钠更有效。用 Marbofloxacin 治疗后,巨噬细胞获得了对感染的抵抗力,并通过 NO 合酶途径增强了抗利什曼尼活性。
|
| 体内研究 (In Vivo) |
治疗剂量的马波沙星治疗不能消除猪肺炎支原体,87.5%至100%的猪在检测结束时仍呈阳性,并且不能有效显着减少临床症状。尽管如此,马波沙星治疗似乎降低了肺部病变评分。在小马组织笼内的金黄色葡萄球菌感染中,每天一次给予马波沙星 6 mg/kg,持续 7 天,对于消除隐蔽场所的金黄色葡萄球菌感染无效。
|
| 动物实验 |
SPF piglets inoculated intratracheally with M. hyopneumoniae strain 116
~2 mg/kg/day Intramuscular injection Tissue cages (TC), implanted subcutaneously in the neck in eight ponies, were inoculated with Staphylococcus aureus (S. aureus) to determine the clinical efficacy of marbofloxacin in the treatment of this infection. From 21 h after inoculation, marbofloxacin (6 mg/kg) was administered intravenously (i.v.) once daily for 7 days. Samples of the tissue cage fluid (TCF) were taken to determine marbofloxacin concentrations (days 1, 3 and 7), using high-pressure liquid chromatography, and numbers of viable bacteria [colony forming units (CFU)] (days 1, 3, 7, 14 and 21). Statistical analysis was used to compare CFU before and after treatment. Clinical signs and CFU were used to evaluate the efficacy of treatment. Although, there was a slight decrease in CFU in all TC initially, the infection was not eliminated by marbofloxacin treatment in any of the ponies and abscesses formed. As the MIC (0.25 microg/mL) did not change during treatment and the concentration of marbofloxacin during treatment (mean concentration in TCF was 0.89 microg/mL on day 1, 0.80 microg/mL on day 3 and 2.77 microg/mL on day 7) was above MIC, we consider that the treatment failure might be attributable to the formation of a biofilm by S. aureus. Based on the present results, i.v. administration of marbofloxacin alone is not suitable for the elimination of S. aureus infections from secluded sites.[2] |
| 药代性质 (ADME/PK) |
Marbofloxacin is a fluoroquinolone antibiotic expected to be effective in the treatment of infections involving gram-negative and some gram-positive bacteria in horses. In order to design a rational dosage regimen for the substance in horses, the pharmacokinetic properties of marbofloxacin were investigated in 6 horses after i.v., subcutaneous and oral administration of a single dose of 2 mg/kg bwt and the minimal inhibitory concentrations (MIC) assessed for bacteria isolated from equine infectious pathologies. The clearance of marbofloxacin was mean +/- s.d. 0.25 +/- 0.05 l/kg/h and the terminal half-life 756 +/- 1.99 h. The marbofloxacin absolute bioavailabilities after subcutaneous and oral administration were 98 +/- 11% and 62 +/- 8%, respectively. The MIC required to inhibit 90% of isolates (MIC90) was 0.027 microg/ml for enterobacteriaceae and 0.21 microg/ml for Staphylococcus aureus. The values of surrogate markers of antimicrobial efficacy (AUIC, Cmax/MIC ratio, time above MIC90) were calculated and the marbofloxacin concentration profiles simulated for repeated administrations. These data were used to determine rational dosage regimens for target bacteria. Considering the breakpoint values of efficacy indices for fluoroquinolones, a marbofloxacin dosage regimen of 2 mg/kg bwt/24 h by i.v., subcutaneous or oral routes was more appropriate for enterobacteriaceae than for S. aureus. [3]
|
| 毒性/毒理 (Toxicokinetics/TK) |
mouse LD50 oral >2 gm/kg United States Patent Document., #4801584
|
| 参考文献 | |
| 其他信息 |
LSM-5799 is a member of quinolines.
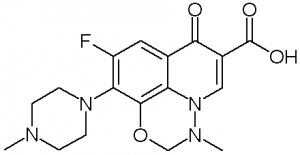
Marbofloxacin is a carboxylic acid, part of the third generation of antibiotic fluoroquinolones. It is used in veterinary medicine. A formulation of marbofloxacin combined with clotrimazole and dexamethasone is available under the name Aurizon. IN THE TITLE COMPOUND, [SYSTEMATIC NAME: 9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-piperazin-1-yl)-7-oxo-7H-pyrido[1,2,3-ij][1,2,4]benzoxadiazine-6-carb-oxy-lic acid], C(17)H(19)FN(4)O(4), the carbonyl and carboxyl groups are coplanar with the quinoline ring, making a dihedral angle of 2.39 (2)°. The piperazine ring adopts a chair conformation and the oxadiazinane ring displays an envelope conformation with the CH(2) group at the flap displaced by 0.650 (2) Å from the plane through the other five atoms. The mol-ecular structure exhibits an S(6) ring motif, owing to an intra-molecular O-H⋯O hydrogen bond. In the crystal, weak C-H⋯F hydrogen bonds link mol-ecules into layers parallel to the ab plane.[1] |
| 分子式 |
C17H19FN4O4
|
|
|---|---|---|
| 分子量 |
362.36
|
|
| 精确质量 |
362.139
|
|
| 元素分析 |
C, 56.35; H, 5.29; F, 5.24; N, 15.46; O, 17.66
|
|
| CAS号 |
115550-35-1
|
|
| 相关CAS号 |
115551-26-3
|
|
| PubChem CID |
60651
|
|
| 外观&性状 |
Light yellow to yellow solid powder
|
|
| 密度 |
1.6±0.1 g/cm3
|
|
| 沸点 |
570.5±60.0 °C at 760 mmHg
|
|
| 熔点 |
268-269ºC
|
|
| 闪点 |
298.8±32.9 °C
|
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
|
| 折射率 |
1.701
|
|
| LogP |
-0.55
|
|
| tPSA |
78.25
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
9
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
26
|
|
| 分子复杂度/Complexity |
636
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
FC1C([H])=C2C(C(C(=O)O[H])=C([H])N3C2=C(C=1N1C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C1([H])[H])OC([H])([H])N3C([H])([H])[H])=O
|
|
| InChi Key |
BPFYOAJNDMUVBL-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C17H19FN4O4/c1-19-3-5-21(6-4-19)14-12(18)7-10-13-16(14)26-9-20(2)22(13)8-11(15(10)23)17(24)25/h7-8H,3-6,9H2,1-2H3,(H,24,25)
|
|
| 化学名 |
7-fluoro-2-methyl-6-(4-methylpiperazin-1-yl)-10-oxo-4-oxa-1,2-diazatricyclo[7.3.1.05,13]trideca-5(13),6,8,11-tetraene-11-carboxylic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|---|
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7597 mL | 13.7984 mL | 27.5969 mL | |
| 5 mM | 0.5519 mL | 2.7597 mL | 5.5194 mL | |
| 10 mM | 0.2760 mL | 1.3798 mL | 2.7597 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|