| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
Kit (IC50 = 200 nM); Lyn B (IC50 = 510 nM); PDGFRα (IC50 = 540 nM); PDGFRβ (IC50 = 800 nM); Abl1 (IC50 = 1.20 μM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:马赛替尼是浓度≤500 nM 的 ATP 竞争性抑制剂。马赛替尼还有效抑制重组 PDGFR 和细胞内激酶 Lyn,并在较小程度上抑制成纤维细胞生长因子受体 3。相比之下,马赛替尼对 Abl 和 c-Fms 的抑制较弱。马赛替尼比伊马替尼更强烈地抑制脱颗粒、细胞因子产生和骨髓肥大细胞迁移。在表达人野生型 Kit 的 Ba/F3 细胞中,马赛替尼抑制 SCF(干细胞因子)诱导的细胞增殖,IC50 为 150 nM,而抑制 IL-3 刺激的增殖的 IC50 约为 >10 µM。在表达 PDGFRα 的 Ba/F3 细胞中,马赛替尼抑制 PDGF-BB 刺激的增殖和 PDGFRα 酪氨酸磷酸化,IC50 为 300 nM。马赛替尼还会抑制肥大细胞瘤细胞系和 BMMC 中 SCF 刺激的人 Kit 酪氨酸磷酸化。马赛替尼抑制 Kit 功能获得突变体,包括 V559D 突变体和 Δ27 小鼠突变体,在 Ba/F3 细胞中 IC50 分别为 3 和 5 nM。马赛替尼抑制肥大细胞瘤细胞系(包括 HMC-1α155 和 FMA3)的细胞增殖,IC50 分别为 10 和 30 nM。马赛替尼抑制两种新型 ISS 细胞系中的细胞生长和 PDGFR 磷酸化,这表明马赛替尼对原发性和转移性 ISS 细胞系均表现出活性,并可能有助于 ISS 的临床管理。激酶测定:96 孔微量滴定板用 0.25 mg/ml 聚(Glu,Tyr 4:1)包被过夜,用 250 µL 洗涤缓冲液(10 mM 磷酸盐缓冲盐水 [pH 7.4] 和 0.05% Tween 20)冲洗两次)并在室温下干燥2小时。测定在室温下进行,最终体积为 50 µL,激酶缓冲液(10 mM MgCl2、1 mM MnCl2、1 mM 原钒酸钠、20 mM HEPES,pH 7.8)中含有 ATP,每次浓度至少为 Km 的两倍酶和适量的重组酶以保证线性反应速率。引入酶后开始反应,并通过向每 5 M 尿素混合物添加一反应体积 (50 μL) 的 100 mM EDTA 来终止反应。将板洗涤三次并与1:30,000辣根过氧化物酶缀合的抗磷酸酪氨酸单克隆抗体一起孵育,然后洗涤三次并与四甲基联苯胺一起孵育。最终反应产物通过 450 nm 分光光度法进行定量。细胞测定:为了测定 Ba/F3 细胞增殖,在 37 °C 下将总共 104 个细胞/孔接种到含有 10% 胎牛血清的 100 μL RPMI 1640 培养基中。这些是否补充或不补充来自 X63-IL-3 细胞的 0.1% 条件培养基或 250 ng/mL 鼠 SCF。激活 Kit 的鼠 SCF 是从产生 SCF 的 CHO 细胞的条件培养基中纯化出来的。细胞与马赛替尼一起在 37 °C 下生长 48 小时,然后与 10 μL/孔的 WST-1 试剂在 37 °C 下孵育 3 小时。使用扫描多孔分光光度计通过 450 nm 处的吸光度来定量形成的甲臜染料的量。没有细胞的空白孔用作分光光度计的背景对照。细胞:表达野生型或突变型人类 Kit、HMC1、HMC-1α155 的 Ba/F3 细胞
|
| 体内研究 (In Vivo) |
马赛替尼在 30 mg/kg 剂量下可抑制表达 Δ27 的 Ba/F3 肿瘤模型中的肿瘤生长并延长中位生存时间,且无心脏毒性或基因毒性。与安慰剂相比,马赛替尼(12.5 mg/kg/d PO)可增加狗的总体 TTP(肿瘤进展时间)。马赛替尼/吉西他滨组合在体外显示出对吉西他滨难治性细胞系 Mia Paca2 和 Panc1 增殖的协同作用,对 NogCID 小鼠中的 Mia Paca-2 胰腺肿瘤的增殖也有较小程度的协同作用。
与安慰剂相比,马西替尼的总TTP从75天增加到118天(P=0.038)。当masitinib用作一线治疗时,这种效果更为明显,中位TTP从75天增加到253天(P=0.001),无论肿瘤是表达突变体(83对未达到[P=.009])还是野生型KIT(66对253[P=.008])。马西替尼通常耐受良好,轻度(I级)或中度(II级)腹泻或呕吐是最常见的不良事件。 结论和临床重要性:马西替尼在延缓复发性或不可切除的II级或III级非转移性MCT犬的肿瘤进展方面是安全有效的[3]。 |
| 酶活实验 |
将 96 孔微量滴定板用 0.25 mg/mL 聚(Glu,Tyr 4:1)包被一整夜。然后用 250 µL 洗涤缓冲液(10 mM 磷酸盐缓冲盐水 [pH 7.4] 和 0.05% Tween 20)冲洗两次,并在室温下干燥两小时。测定在室温下进行,最终体积为 50 µL 激酶缓冲液(10 mM MgCl2、1 mM MnCl2、1 mM 原钒酸钠、20 mM HEPES ,pH 7.8),含有重组酶和 ATP,每种酶的浓度至少是 Km 的两倍,以保证线性反应速率。添加酶以启动反应,并通过每 5mol/Lurea 混合物添加一反应体积 (50 μL) 的 100 mM EDTA 来停止反应。将板洗涤三次,然后与四甲基联苯胺和 1:30,000 辣根过氧化物酶缀合的抗磷酸酪氨酸单克隆抗体一起孵育。使用分光光度法在 450 nm 处测量最终反应产物。
重组蛋白激酶的体外检测[1] 补充方法中提供了重组人KIT细胞内结构域和其他蛋白激酶(包括Lyn、血小板衍生生长因子受体β、表皮生长因子受体、成纤维细胞生长因子受体1、Src、HCK、PYK、FES、Btk、Bmx、c-Ret、c-Fms、Syk和c-Met)产生的全部细节(见支持信息;方法S1)。用Proqinase对ABL1、Akt1、蛋白激酶C-α、胰岛素样生长因子受体1和Pim1进行了实验。所有其他重组蛋白激酶都是在内部使用酶联免疫测定法进行的;补充方法中提供了实验细节(见支持信息;方法S1)。 |
| 细胞实验 |
将微量滴定板在 37°C 下以 104 细胞/孔接种到 100 μL 含有 10% 胎牛血清的 RPMI 1640 培养基中,以进行 Ba/F3 细胞增殖测定。向其中添加或不添加 250 ng/mL 的鼠 SCF 或来自 X63-IL-3 细胞的 0.1% 条件培养基。从产生 SCF 的 CHO 细胞的条件培养基中纯化得到的鼠 SCF 可以激活 Kit。将马赛替尼生长的细胞与 WST-1 试剂(10 μL/孔)一起在 37°C 下孵育 48 小时,持续三小时。使用扫描多孔分光光度计,甲臜染料在 450 nm 处的吸光度表明其形成量。分光光度计的背景对照是不含细胞的空白孔。
如Royer等人之前所述(见支持信息;方法S1),对通过长期培养从正常脐带血纯化的CD34+祖细胞产生的CBMC进行了评估,评估了masitinibb和伊马替尼对人类肥大细胞脱颗粒反应和细胞因子产生(TNF-α释放)的影响。收获培养的细胞,在完全IMDM培养基中洗涤,并在不同浓度的masitinib或伊马替尼中孵育1小时。通过用1µg/ml的山羊抗人IgE刺激CBMC 30分钟或4小时,分别进行β-己糖胺酶释放和TNF-α释放的测定。在上清液和超声处理的细胞颗粒中测量β-己糖胺酶,并计算其净释放量。对于TNF-α测定,通过离心收集无细胞上清液,并在-80°C下冷冻,直至根据制造商的说明使用特定的ELISA试剂盒测定介质含量。所有测定均重复进行,每个孔重复计数两次。结果以β-己糖胺酶释放和TNF-α释放的抑制百分比表示,相对于受刺激的未经治疗的CBMC(即100%的刺激)。 血小板源性生长因子受体(PDGFR)的失调可能在猫注射部位肉瘤(ISS)细胞的生长和存活中发挥作用Masitinib是一种酪氨酸激酶抑制剂,被批准用于治疗犬肥大细胞肿瘤,对PDGFR信号通路具有高度选择性,可能为这种疾病提供一种新的治疗方法。研究了masitinib对两种新型ISS细胞系的生长、凋亡和PDGFR信号传导的体外影响。在来源于原发性ISS肿瘤(JB)和相应的经组织学证实的ISS肺转移(JBLM)的细胞系中,通过蛋白质印迹证实了PDGFR的表达。马西替尼抑制了两种细胞系的细胞生长和PDGFR磷酸化。与调节配体诱导的PDGFR自磷酸化相比,抑制生长需要更高的药物浓度。这些体外数据表明,masitinib对原发性和转移性ISS细胞系都显示出活性,可能有助于ISS的临床管理[2]。 |
| 动物实验 |
At 7 weeks old, male Nog-SCID mice are housed in a pathogen-free environment with filtered water and food available at all times. They experience a 12-hour light/12-hour dark cycle. According to the above description, Mia Paca-2 cells are cultured. Mice are given an injection into the right flank at day 0 (D0) containing 107 Mia Paca-2 cells in 200 µL PBS. After a tumor reaches the target size of approximately 200 mm3, it is allowed to grow for 1.5 to 4 weeks. In order to ensure that the mean body weight and tumor volume of each treatment group are well matched, animals are divided into four groups by day 28 (n = 7–8). The animals receive treatment for a maximum of four weeks, following which they are sacrificed. The treatments were as follows: a) daily gavage with 100 mg/kg masitinib; b) intraperitoneal (i.p.) injection of 50 mg/kg gemcitabine twice a week; c) daily gavage with 100 mg/kg masitinib; or d) a combination of daily gavage with 100 mg/kg masitinib and i.p. injection of 50 mg/kg gemcitabine twice a week. Callipers are used to measure the size of tumors, and the formula volume=(length × width2)/2 is used to estimate the tumor volume. (100) × (median tumor volume of treated group)/(median tumor volume of control group) is the formula for the tumor growth inhibition ratio.
In vivo assays with Ba/F3 Δ27 tumour model [1] Female MBRI Nu/Nu mice (7 weeks old) were housed under specific pathogen-free conditions at 20±1°C with a 12 hours light/12 hours dark cycle and ad libitum access to food and filtered water. The mice were allowed to acclimatise to the study conditions for 10 to 20 days prior to experiments. The Δ27-expressing Ba/F3 cells were grown in RPMI 1640 medium supplemented with glutamax-1 and 10% foetal bovine serum at 37°C in a humidified atmosphere containing 5% CO2. The cells were centrifuged and resuspended at 5×106 or 7.5×106 cells/ml in phosphate-buffered saline. Mice were treated with 5 Gy of gamma radiation and after 24 hours they were injected in the right flank with 1.5×106 Δ27 Ba/F3 cells. When tumour growth had reached the desired size, mice were allocated into treatment groups ensuring that there was no statistical difference between each group's mean body weight and tumour volume. For all animals, body weight was measured on the day of injection and every 5 days thereafter, with the tumour's size measured via callipers every 5 days during the treatment period for estimation of tumour volume. During the predose period and for 2 weeks post-treatment, the animals were checked for mortality or signs of morbidity once a day, increasing to twice a day checks during the treatment period. Background: Activation of the KIT receptor tyrosine kinase is associated with the development of canine mast cell tumors (MCT). [3] Hypothesis/objective: To evaluate the efficacy of masitinib, a potent and selective inhibitor of KIT, in the treatment of canine MCT. [3] Animals: Two hundred and two client-owned dogs with nonmetastatic recurrent or nonresectable grade II or III MCT. [3] Methods: Double-blind, randomized, placebo-controlled phase III clinical trial. Dogs were administered masitinib (12.5 mg/kg/d PO) or a placebo. Time-to-tumor progression (TTP), overall survival, objective response at 6 months, and toxicity were assessed. [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
Furthermore, in an intraperitoneal model, masitinib significantly enhanced survival with no indication of general toxicity, as indicated by a lack of weight loss at the administered doses. [1]
|
| 参考文献 |
|
| 其他信息 |
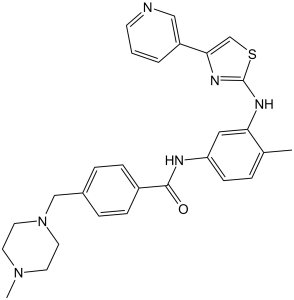
Masitinib is a member of the class of benzamides that is the carboxamide resulting from the formal condensation of the carboxy group of 4-[(4-methylpiperazin-1-yl)methyl]benzoic acid with the primary amino group of 4-methyl-N(3)-[4-(pyridin-3-yl)-1,3-thiazol-2-yl]benzene-1,3-diamine. It is a highly selective oral tyrosine kinase inhibitor. It has a role as a tyrosine kinase inhibitor, an antineoplastic agent and an antirheumatic drug. It is a N-alkylpiperazine, a member of 1,3-thiazoles, a member of pyridines and a member of benzamides.
Masitinib is a tyrosine-kinase inhibitor used in the treatment of mast cell tumors in dogs. It has been available in Europe since 2009, under the brand name Masivet. In the USA it is distributed under the name Kinavet and has been available for veterinaries since 2011. Masitinib is a multi-targeted protein tyrosine kinase inhibitor, with potential antineoplastic activity. Upon administration, masitinib selectively binds to and inhibits both the wild-type and mutated forms of the stem cell factor receptor (c-Kit; SCFR); platelet-derived growth factor receptor (PDGFR); fibroblast growth factor receptor 3 (FGFR3); and, to a lesser extent, focal adhesion kinase (FAK). As a consequence, tumor cell proliferation may be inhibited in cancer cell types that overexpress these receptor tyrosine kinases (RTKs). See also: Masitinib Mesylate (annotation moved to). Drug Indication Treatment of amyotrophic lateral sclerosis. Treatment of mastocytosis Treatment of non resectable locally advanced or metastatic pancreatic cancer Treatment of unresectable and/or metastatic malignant gastrointestinal stromal tumour (GIST). Background[1] The stem cell factor receptor, KIT, is a target for the treatment of cancer, mastocytosis, and inflammatory diseases. Here, we characterise the in vitro and in vivo profiles of masitinib (AB1010), a novel phenylaminothiazole-type tyrosine kinase inhibitor that targets KIT. [1] Methodology/Principal Findings[1] In vitro, masitinib had greater activity and selectivity against KIT than imatinib, inhibiting recombinant human wild-type KIT with an half inhibitory concentration (IC50) of 200±40 nM and blocking stem cell factor-induced proliferation and KIT tyrosine phosphorylation with an IC50 of 150±80 nM in Ba/F3 cells expressing human or mouse wild-type KIT. Masitinib also potently inhibited recombinant PDGFR and the intracellular kinase Lyn, and to a lesser extent, fibroblast growth factor receptor 3. In contrast, masitinib demonstrated weak inhibition of ABL and c-Fms and was inactive against a variety of other tyrosine and serine/threonine kinases. This highly selective nature of masitinib suggests that it will exhibit a better safety profile than other tyrosine kinase inhibitors; indeed, masitinib-induced cardiotoxicity or genotoxicity has not been observed in animal studies. Molecular modelling and kinetic analysis suggest a different mode of binding than imatinib, and masitinib more strongly inhibited degranulation, cytokine production, and bone marrow mast cell migration than imatinib. Furthermore, masitinib potently inhibited human and murine KIT with activating mutations in the juxtamembrane domain. In vivo, masitinib blocked tumour growth in mice with subcutaneous grafts of Ba/F3 cells expressing a juxtamembrane KIT mutant. [1] Conclusions[1] Masitinib is a potent and selective tyrosine kinase inhibitor targeting KIT that is active, orally bioavailable in vivo, and has low toxicity.[1] Masitinib mesylate (AB1010) is a novel potent and selective tyrosine kinase inhibitor, targeting mainly wild-type and mutated c-Kit receptor (c-KitR), Platelet Derived Growth Factor Receptor-alfa/beta (PDGFRa/ß), Lymphocyte-specific kinase (Lck), Lck/Yes-related protein (LYn), Fibroblast Growth Factor Receptor 3 (FGFR3) and Focal Adhesion Kinase (FAK). It is the first anticancer therapy approved in veterinary medicine for the treatment of unresectable canine mast cell tumors (CMCTs), harboring activating c-KitR mutations, at dose of 12.5mg/kg once daily. Considering its anti-proliferative action, principally given by inhibiting the MCs c-KitR anti-angiogenic pathway that leads cancer progression, and its role as chemosensitizer, masitinib is under clinical investigation in several human malignancies (Gastro-Intestinal Stromal Tumors, acute myeloid leukemia, systemic mastocytosis, pancreatic cancer, multiple myeloma, non-small cell lung cancer, melanoma, ovarian and prostate cancer), which are characterized by similar canine c-KIT proto-oncogene mutations. Here, we analyze masitinib structure activity, its pharmacokinetics compared to imatinib, the c-KitR pathway referring to the most frequent c-KIT mutations sensitive or resistant to this novel drug compared to imatinib, and masitinib safety profile. We, also, explore preclinical and clinical (completed and ongoing) trials with the aim to emphasize as this recent anti-angiogenic therapy, at first approved in CMCTs and, currently in development for the treatment of several human neoplasms, could be represent a milestone in translational oncology, in which the murine experimental model of cancer research could be integrated by canine spontaneous tumor model. [4] |
| 分子式 |
C28H30N6OS
|
|---|---|
| 分子量 |
498.64
|
| 精确质量 |
498.22
|
| 元素分析 |
C, 67.44; H, 6.06; N, 16.85; O, 3.21; S, 6.43
|
| CAS号 |
790299-79-5
|
| 相关CAS号 |
Masitinib mesylate;1048007-93-7
|
| PubChem CID |
10074640
|
| 外观&性状 |
Off-white to pale yellow solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 熔点 |
90-95ºC
|
| 折射率 |
1.682
|
| LogP |
2.88
|
| tPSA |
101.63
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
36
|
| 分子复杂度/Complexity |
696
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C(C1C=CC(CN2CCN(C)CC2)=CC=1)NC1C=C(NC2SC=C(C3C=CC=NC=3)N=2)C(C)=CC=1
|
| InChi Key |
WJEOLQLKVOPQFV-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C28H30N6OS/c1-20-5-10-24(16-25(20)31-28-32-26(19-36-28)23-4-3-11-29-17-23)30-27(35)22-8-6-21(7-9-22)18-34-14-12-33(2)13-15-34/h3-11,16-17,19H,12-15,18H2,1-2H3,(H,30,35)(H,31,32)
|
| 化学名 |
4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-yl-1,3-thiazol-2-yl)amino]phenyl]benzamide
|
| 别名 |
AB-1010; AB 1010; 790299-79-5; Masitinib [INN]; AB 1010; AB1010; Masitinib; Brand name: Kinavet; Masivet.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.01 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.01 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.01 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 4% DMSO+30% PEG 300+5% Tween 80+ddH2O: 30mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0055 mL | 10.0273 mL | 20.0545 mL | |
| 5 mM | 0.4011 mL | 2.0055 mL | 4.0109 mL | |
| 10 mM | 0.2005 mL | 1.0027 mL | 2.0055 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05441488 | Recruiting | Drug: Placebo Drug: Masitinib (4.5) |
Progressive Multiple Sclerosis | AB Science | June 28, 2022 | Phase 3 |
| NCT05047783 | Recruiting | Drug: Masitinib Mesylate Drug: Placebo |
Covid19 SARS-CoV2 Infection |
AB Science | November 23, 2021 | Phase 2 |
| NCT05564169 | Not yet recruiting | Drug: Placebo Drug: Masitinib (4.5) |
Alzheimer Disease | AB Science | January 2024 | Phase 3 |
| NCT04333108 | Recruiting | Drug: Masitinib Other: Placebo |
Indolent Systemic Mastocytosis | AB Science | July 1, 2020 | Phase 3 |
| NCT04622865 | Recruiting | Drug: Masitinib Drug: Isoquercetin |
SARS-CoV 2 COVID-19 |
AB Science | June 1, 2020 | Phase 2 |
Masitinib inhibition of KIT in intact cells. PLoS One. 2009; 4(9): e7258. |
Masitinib inhibits tumour growth in vivo. PLoS One. 2009; 4(9): e7258. |
Effect of masitinib on BCR-ABL and PDGFRα. |