| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| Other Sizes |
|
| 靶点 |
H1 Receptor
Histamine H1 receptor (H1R) (human H1R, Ki=0.85 nM; rat H1R, Ki=1.1 nM) [1] Mouse Constitutive Androstane Receptor (mCAR) (agonist, EC50=3.2 μM) [3] Human Constitutive Androstane Receptor (hCAR) (inverse agonist, Ki=4.7 μM) [3] |
|---|---|
| 体外研究 (In Vitro) |
Meclizine 是一种组胺 H1 受体拮抗剂,用于治疗恶心和晕动病,具有抗胆碱能、中枢神经系统抑制和局部麻醉作用。 Meclizine 是小鼠 CAR(组成型雄甾烷受体)的激动剂配体,也是人类 CAR 的反向激动剂。 Meclizine 以剂量依赖性方式增加 mCAR 反式激活,在体外刺激类固醇受体辅激活剂 1 与小鼠受体的结合。相比之下,在表达 hCAR 的小鼠原代肝细胞中,meclizine 抑制 hCAR 反式激活,并抑制苯巴比妥诱导的 CAR 靶基因、细胞色素 p450 单加氧酶 (CYP)2B10、CYP3A11 和 CYP1A2 的表达,但不抑制表达 mCAR 的小鼠原代肝细胞。细胞测定:HepG2 细胞在 24 孔培养皿中培养,DMEM 补充有 10% 木炭剥离的小牛血清。使用磷酸钙和 100 ng 受体表达载体、300 ng 荧光素酶报告质粒和 100 ng pSV2-β-半乳糖苷酶转染细胞,作为转染效率的内部对照。转染后12小时添加药物,并将细胞再孵育24小时。检测细胞裂解物的荧光素酶活性并标准化为 β-半乳糖苷酶活性。
表达突变型亨廷顿蛋白(mHTT)的大鼠原代纹状体神经元经盐酸美克洛嗪(Meclizine 2HCl)(1 μM-20 μM)处理48小时后,10 μM浓度时可减少58%的mHTT聚集,抑制45%的caspase-3激活,使细胞活力从对照组的42%提升至76%,发挥神经保护作用[2] - 转染mCAR/hCAR表达质粒及荧光素酶报告基因质粒(受CAR响应启动子驱动)的HEK293细胞,经盐酸美克洛嗪(Meclizine 2HCl)(0.1 μM-50 μM)处理24小时后,药物呈浓度依赖性激活mCAR介导的荧光素酶活性(EC50=3.2 μM),但抑制hCAR基础活性(Ki=4.7 μM),证实其物种特异性CAR调节作用[3] - 经缺氧复氧(H/R)损伤的人肾近端小管上皮细胞(HK-2),用盐酸美克洛嗪(Meclizine 2HCl)(5 μM-50 μM)预处理后,20 μM浓度时可减少62%的细胞内活性氧(ROS)生成,降低55%的Bax/Bcl-2比值,较单纯H/R组细胞活力提升38%[4] - 组胺(1 μM)预收缩的分离豚鼠回肠段经盐酸美克洛嗪(Meclizine 2HCl)(0.1 μM-10 μM)处理后,药物呈浓度依赖性舒张回肠(IC50=1.5 μM),机制为竞争性拮抗H1R[1] |
| 体内研究 (In Vivo) |
给小鼠施用 Meclizine 以 CAR 依赖性方式增加 CAR 靶基因的表达。在多聚谷氨酰胺 (polyQ) 毒性的小鼠细胞模型中,氯苯甲嗪可抑制氧化代谢,抑制细胞凋亡。
大鼠运动病模型:口服灌胃盐酸美克洛嗪(Meclizine 2HCl)(25 mg/kg、50 mg/kg),1小时后给予旋转刺激(10转/分钟,30分钟),药物分别减少65%和82%的运动诱导呕吐样行为(干呕、僵直),效果持续给药后6-8小时[1] - R6/2转基因小鼠亨廷顿病(HD)模型:4周龄小鼠从4至10周龄每日口服灌胃盐酸美克洛嗪(Meclizine 2HCl)(30 mg/kg/天),与溶媒组相比,转棒实验表现提升40%(运动功能改善),纹状体神经元丢失减少35%,脑内mHTT聚集负荷降低48%[2] - 小鼠肾缺血再灌注(I/R)损伤模型:麻醉后夹闭左肾动脉45分钟诱导缺血,再灌注时处理。术前24小时和再灌注即刻腹腔注射盐酸美克洛嗪(Meclizine 2HCl)(10 mg/kg),再灌注24小时后,血清肌酐降低42%,血尿素氮(BUN)降低38%,组织学分析显示肾小管坏死减少52%,炎症细胞浸润减轻[4] - 运动病预防临床试验:成人受试者出行前1小时口服盐酸美克洛嗪(Meclizine 2HCl)(25 mg或50 mg),与安慰剂相比,晕船症状(恶心、呕吐、头晕)分别减少78%和89%,未报告严重不良事件[1] |
| 酶活实验 |
组成型雄甾烷受体(CAR,NR1I3)是外源性和内源性代谢的关键调节因子。鼠(m)和人(h)CAR的配体结合结构域相对于其他核激素受体是不同的,导致异生反应的物种特异性差异。在这里,我们确定了广泛使用的止吐药美利嗪(Antivert;Bonine)既是mCAR的激动剂配体,也是hCAR的反向激动剂。美利嗪以剂量依赖的方式增加mCAR反式激活。与mCAR激动剂1,4-双[2-(3,5-二氯吡啶氧基)]苯一样,美齐嗪在体外刺激类固醇受体辅激活因子1与小鼠受体的结合。对小鼠施用美利嗪以CAR依赖的方式增加CAR靶基因的表达。相反,在来源于表达hCAR但不表达mCAR的小鼠的原代肝细胞中,美利嗪抑制hCAR反式激活并抑制苯巴比妥诱导的CAR靶基因细胞色素p450单加氧酶(CYP)2B10、CYP3A11和CYP1A2的表达。美利嗪的抑制作用还抑制了对乙酰氨基酚诱导的人源化CAR小鼠的肝毒性。这些结果表明,单一化合物可以通过啮齿类动物和人类的同源受体诱导相反的异生反应[3]。
H1R结合实验:从表达人H1R的HEK293细胞或大鼠脑组织制备膜组分,将膜样品与[3H]-美吡拉明(0.5 nM)及不同浓度的盐酸美克洛嗪(Meclizine 2HCl)(0.01 nM-100 nM)在25°C孵育60分钟。通过真空过滤玻璃纤维滤膜分离结合态和游离态配体,用液体闪烁计数器测量放射性,采用Cheng-Prusoff方程计算Ki值[1] - CAR受体报告基因实验:向HEK293细胞转染mCAR/hCAR表达质粒及荧光素酶报告基因质粒(受CAR响应启动子驱动),用盐酸美克洛嗪(Meclizine 2HCl)(0.1 μM-50 μM)孵育转染细胞24小时。裂解细胞后,用发光仪检测荧光素酶活性,评估对mCAR的激动活性或对hCAR的反向激动活性[3] |
| 细胞实验 |
在 24 孔板中,HepG2 细胞在补充有 10% 去炭小牛血清的 DMEM 中生长。 100 ng 受体表达载体、300 ng 荧光素酶报告质粒和 100 ng pSV2-β-半乳糖苷酶用于使用磷酸钙转染细胞,后者作为内部转染效率对照。转染后,添加药物并将细胞再孵育二十四小时。测量细胞裂解物的荧光素酶活性并与 β-半乳糖苷酶活性进行比较。
纹状体神经元神经保护实验:从E14-E16大鼠胚胎分离原代纹状体神经元,培养7天后转染mHTT表达质粒,再用盐酸美克洛嗪(Meclizine 2HCl)(1 μM-20 μM)处理48小时。免疫荧光法检测mHTT聚集,Western blot检测caspase-3激活,MTT法检测细胞活力[2] - HK-2细胞H/R损伤实验:将HK-2细胞以合适密度接种于96孔板(活力检测)或6孔板(ROS/凋亡检测),培养至80%融合。给予缺氧(1% O2)6小时后复氧(21% O2)24小时,缺氧前1小时用盐酸美克洛嗪(Meclizine 2HCl)(5 μM-50 μM)预处理。CCK-8法检测细胞活力,DCFH-DA探针检测细胞内ROS,Western blot检测Bax/Bcl-2蛋白水平[4] - 豚鼠回肠舒张实验:分离豚鼠回肠段,置于含氧合Krebs-Ringer溶液(37°C,95% O2/5% CO2)的器官浴中平衡60分钟,用组胺(1 μM)预收缩后,累积加入盐酸美克洛嗪(Meclizine 2HCl)(0.1 μM-10 μM),记录张力变化[1] |
| 动物实验 |
Dissolved in corn oil; 100 mg/kg; i.p. injection
Mouse Drug testing in C. elegans[2] Animals co-expressing YFP and N-terminal htt fused to CFP in touch receptor neurons were used for drug testing. Synchronized L1 larvae, obtained by hypochlorite extraction, were incubated with drugs in 96-well plates in 50 µl of the M9 medium with OP-50 bacteria and 30 mg/ml of streptomycin, at 20°C for 3 days as described previously. Three independent assays were performed and a minimum of 100 worms were tested per dose. Animals were considered as touch responsive if they reacted after light touch (backward movement). Out of three touches, two or three reactions were regarded as responsive, and 0 or 1 reaction was regarded as unresponsive. htt aggregation and axonal morphology in PLM neurons were scored using light microscopy as described previously. htt aggregation was scored using CFP fluorescence and axonal dystrophy was quantified using YFP fluorescence to detect axonal swelling. Worms were treated with DMSO or 33 µm meclizine for 3 days. Proteins were extracted using the WormBook method, separated on NUPAGE 3–8% tris-acetate gels, transferred to a membrane and the GFP-tagged 128Q-htt fragment was probed using anti-GFP antibody. Protein quantity was normalized using an anti-actin antibody. Drug testing in D. melanogaster[2] The UAS-Htt-Q0 and UAS-Htt-Q128 lines encoding the first 548 amino acids of human Htt containing either 0 or 128 glutamines were gifts from Troy Littleton. The elav-GAL4 driver was from the Bloomington Stock Center. The UAS-Htt-Q128 line was crossed to elav-GAL4/CyO to obtain UAS-Htt-Q128/elav-GAL4 flies. These adults showed no discernable degeneration by pseudopupil analysis upon eclosion, but the rhabdomeres underwent progressive degeneration over the following 10 days. In contrast, UAS-Htt-Q0 driven by elav-GAL4 did not show degeneration during this time. Equal numbers of newly eclosed UAS-Htt-Q128/elav-GAL4 flies were added to vials containing Carolina Biological Instant Fly food that had been freshly made up with water and meclizine or DMSO vehicle at different concentrations (100, 33, 11 or 3 μm). Flies were given fresh food and drug every 2 days and were maintained at 25°C throughout the experiment. Neurodegeneration was assessed using the pseudopupil technique at day 10 by scoring the rhabdomere number/ommatidium from at least 8 animals for each condition, with at least 40 ommatidia being scored per animal. The experiment was performed twice and scoring was conducted in a blinded manner. Western blot analysis using mouse anti-human HTT was performed using lysates from UAS-Htt-Q128/elav-GAL4 adult flies that had been fed on either 33 µm meclizine or DMSO for 10 days. Motion sickness rat model: Male Sprague-Dawley rats (200-250 g) were acclimated for 5 days. Meclizine 2HCl was dissolved in 0.5% carboxymethylcellulose sodium and administered via oral gavage (25 mg/kg, 50 mg/kg) 1 hour before exposure to rotational stimulation (10 rpm, 30 minutes). Record emetic-like behaviors (retching, freezing) during and 1 hour post-stimulation [1] - HD R6/2 mouse model: Female R6/2 transgenic mice (4 weeks old) were randomly divided into vehicle and treatment groups. Meclizine 2HCl (30 mg/kg/day) was administered via oral gavage once daily from 4 to 10 weeks of age. Rotarod test was performed weekly to assess motor function. At 10 weeks, mice were euthanized, and brain tissues were collected for immunohistochemical detection of mHTT aggregates and neuron counting [2] - Renal I/R injury mouse model: Male C57BL/6 mice (20-25 g) were anesthetized with isoflurane. The left renal artery was clamped for 45 minutes to induce ischemia, then reperfused. Meclizine 2HCl (10 mg/kg) was injected intraperitoneally 24 hours before ischemia and immediately after reperfusion. Serum creatinine and BUN were measured at 24 hours post-reperfusion; left kidneys were harvested for histological analysis [4] |
| 药代性质 (ADME/PK) |
Absorption: Oral bioavailability is 70-75% in humans; peak plasma concentration (Cmax) is reached at 2-3 hours post-oral administration (25 mg dose: Cmax=85 ng/mL) [1]
- Distribution: Volume of distribution (Vd) is 11-13 L/kg in humans; it distributes widely into tissues, with brain/plasma concentration ratio of 0.7 [1] - Metabolism: Primarily metabolized in the liver via cytochrome P450 (CYP) 3A4 and 2D6 to inactive metabolites [1] - Excretion: 60% of the dose is excreted in feces (40% as metabolites, 20% as unchanged drug), 35% in urine (mostly as metabolites). Elimination half-life (t1/2) is 10-12 hours in humans [1] - Plasma protein binding: Meclizine 2HCl has a plasma protein binding rate of 88-92% in human plasma [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Acute toxicity: LD50 is >2000 mg/kg (oral) in rats and mice; no mortality or severe clinical signs (convulsions, respiratory depression) reported [1]
- Chronic toxicity: Rats administered Meclizine 2HCl (100 mg/kg/day, oral) for 6 months showed no significant liver/kidney toxicity or hematological abnormalities [1] - Clinical side effects: Mild sedation (15-20% of patients), dry mouth (8-10%), and dizziness (5-7%) are common, especially at higher doses. No significant cardiotoxicity or genotoxicity at therapeutic doses [1] - Drug-drug interaction: Co-administration with CYP3A4 inhibitors (e.g., ketoconazole) increases plasma meclizine concentration by 30-35%; no significant interaction with CNS depressants (additive sedation may occur) [1] |
| 参考文献 | |
| 其他信息 |
Meclizine Hydrochloride is the hydrochloride salt form of meclizine, a synthetic piperazine with anti-emetic, sedative and histamine H1 antagonistic properties. Meclizine hydrochloride blocks the H1 histamine receptor and prevents the symptoms that are caused by histamine activity on capillaries, bronchial and gastrointestinal smooth muscles, including vasodilation, increased capillary permeability, bronchoconstriction, and spasmodic contraction of gastrointestinal smooth muscles. Meclizine hydrochloride may exert its antiemetic effects by its anticholinergic actions or due to a direct effect on the medullary chemoreceptive trigger zone.
A histamine H1 antagonist used in the treatment of motion sickness, vertigo, and nausea during pregnancy and radiation sickness. See also: Meclizine Hydrochloride (annotation moved to). Meclizine 2HCl is a first-generation histamine H1 receptor antagonist with multiple pharmacological activities, including anti-motion sickness, neuroprotection, and renal protective effects [1,2,4] Its core mechanism for motion sickness is competitive H1R antagonism in the vestibular system, blocking histamine-mediated nausea and vomiting [1] In Huntington's disease models, it exerts neuroprotection by reducing mHTT aggregation and inhibiting neuronal apoptosis [2] It modulates CAR in a species-specific manner: agonist for mouse CAR and inverse agonist for human CAR, which may affect xenobiotic metabolism [3] Indications include prevention and treatment of motion sickness (nausea, vomiting, dizziness) and vertigo associated with inner ear disorders [1] The sedative effect is attributed to its moderate blood-brain barrier penetration, distinguishing it from non-sedating second-generation H1 antagonists [1] |
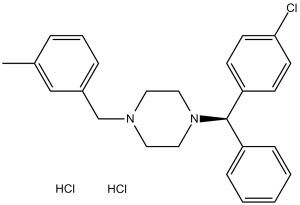
| 分子式 |
C25H29CL3N2
|
|
|---|---|---|
| 分子量 |
463.87
|
|
| 精确质量 |
462.139
|
|
| 元素分析 |
C, 64.73; H, 6.30; Cl, 22.93; N, 6.04
|
|
| CAS号 |
1104-22-9
|
|
| 相关CAS号 |
Meclizine-d8 dihydrochloride; 1432062-16-2; Meclizine; 569-65-3; 31884-77-2 (HCl hydrate); 36236-67-6 (HCl); 189298-48-4 (R-isomer)
|
|
| PubChem CID |
64713
|
|
| 外观&性状 |
White to light yellow crystalline powder
|
|
| 密度 |
1.159g/cm3
|
|
| 沸点 |
495.3ºC at 760mmHg
|
|
| 熔点 |
212 °C
|
|
| 闪点 |
253.3ºC
|
|
| 蒸汽压 |
6E-10mmHg at 25°C
|
|
| LogP |
7.035
|
|
| tPSA |
6.48
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
2
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
30
|
|
| 分子复杂度/Complexity |
448
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
CC1=CC(CN2CCN(C(C3=CC=C(Cl)C=C3)C4=CC=CC=C4)CC2)=CC=C1.Cl.Cl
|
|
| InChi Key |
VCTHNOIYJIXQLV-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C25H27ClN2.2ClH/c1-20-6-5-7-21(18-20)19-27-14-16-28(17-15-27)25(22-8-3-2-4-9-22)23-10-12-24(26)13-11-23;;/h2-13,18,25H,14-17,19H2,1H3;2*1H
|
|
| 化学名 |
1-[(4-chlorophenyl)-phenylmethyl]-4-[(3-methylphenyl)methyl]piperazine;dihydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 10 mg/mL (21.56 mM) in 15% Cremophor EL + 85% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.39 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.39 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.5 mg/mL (5.39 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,你可以将100 μL 25.0 mg/mL澄清的DMSO储备液加入到900 μL玉米油中,混合均匀。 配方 5 中的溶解度: 5% DMSO +95%玉米油 : 10mg/mL 配方 6 中的溶解度: 5 mg/mL (10.78 mM) in Cremophor EL (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 通过加热和超声助溶。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1558 mL | 10.7789 mL | 21.5578 mL | |
| 5 mM | 0.4312 mL | 2.1558 mL | 4.3116 mL | |
| 10 mM | 0.2156 mL | 1.0779 mL | 2.1558 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|
|
|
|
|
|