| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|

| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
... A single oral dose of 0.025 mCi (0.38 mg) [14C]melamine /was administered/ to adult male Fischer 344 rats. Within the first 24 hr, 90% of the administered dose was excreted in the urine. Negligible radioactivity appeared in breath and feces. There was little difference in blood, liver or plasma concentrations of 14C, suggesting that melamine distributes in body water. The only organs showing radioactivity levels much higher than plasma were the kidney and bladder. The bladder level was by far the highest, a finding probably due either to back diffusion from urine or to contamination of bladder tissue with urine. Virtually no residual radioactivity was observed in tissues examined at 24 hr or later. The elimination-phase half-life calculated from plasma data, 2.7 hr, was in good agreement with the urinary-excretion half-life of 3.0 hr. The renal clearance of melamine was 2.5 mL/min. ... Following oral administration of 250 mg/kg melamine to rats, 50% of the mother compound was excreted with the urine within 6 hrs. ... Crystals found in the urine were composed of dimelamine monophosphate, amounting to nearly 20% of the administered dose. After feeding melamine to dogs, 60 - 86.5% of the mother compound was recovered in the urine within 24 hrs. ... Doses of 2.4 g/kg cause diuresis & elimination of fine crystals of dimelamine monophosphate in urine. After administration of a single oral dose of 0.38 mg (14)C-melamine to adult male Fischer 344/N rats, 90% of the administered dose was excreted in the urine within the first 24 hours. Negligible radioactivity was detected in exhaled air and feces; and radioactivity was concentrated in the kidney and bladder. Virtually no residual radioactivity was observed in tissue after 24 hours or more. Chromatography of the radioactivity found in plasma or urine indicated that melamine is not metabolized in rats. Metabolism / Metabolites Toxicokinetic studies in rats given 14C-labelled cyromazine as single and repeated oral doses showed that the active substance is rapidly and almost completely absorbed from the gastrointestinal tract and distributed to all organs and tissues. ... Cyromazine was incompletely metabolized, essentially by methylation, hydroxylation or Ndealkylation. The major component present was cyromazine, which accounted for 71-72% of the radiolabel; a further 7% was attributable to melamine, 8-11% to hydroxy-cyromazine and methylcyromazine. ... A single oral dose of 0.025 mCi (0.38 mg) [14C]melamine /was administered/ to adult male Fischer 344 rats. ... Radioactivity in plasma or urine co-chromatographed with that of the dosing solution, indicating that melamine is not metabolized in the male Fischer 344 rat. Crystalluria was due to excretion of dimelamine-monophosphate crystals. Melamine is not metabolized and is rapidly eliminated via urine in a study with oral application to rats. (L1777) Biological Half-Life ... A single oral dose of 0.025 mCi (0.38 mg) [14C]melamine /was administered/ to adult male Fischer 344 rats. ... The elimination-phase half-life calculated from plasma data, 2.7 hr, was in good agreement with the urinary-excretion half-life of 3.0 hr. ... |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION: Melamine is a monoclinic prismatic substance that is slightly soluble in water and ethanol. It is insoluble in diethyl ether. Melamine forms synthetic resins with formaldehyde. It is used in the manufacture of melamine resins, laminates, surface coating resins, plastic molding compounds, textile resins, bonding resins, gypsum melamine resin mixtures, orthopedic casts, rubber additives and paper products. HUMAN EXPOSURE: Occupational exposure to melamine may occur during its production and use in manufacture of synthetic resins with formaldehyde. ANIMAL STUDIES: Melamine was tested for its carcinogenicity by oral diet in mice and rats and for initiating activity by skin application in mice. No neoplasm related to treatment was observed after oral administration in mice. Male rats fed diets containing melamine developed transitional cell tumors in the urinary bladder, with one exception; all tumor bearing animals had bladder stones probably containing melamine. The incidence of urinary bladder hyperplasia associated with toxicity in male mice treated with melamine in the diet was observed. Groups of 20 male Fisher 344 rats, six weeks old were fed diets containing melamine with a purity of 99.4% with or without 10% NaCl for a total of 36 weeks and were sacrificed at week 40. Urinary bladder carcinomas were observed in all dose groups given melamine alone and melamine with NaCl. No carcinomas were observed in the melamine and NaCl groups. The incidences of papillomas were significantly decreased by NaCl. In contrast to the incidence of papillomas in the group given high dose melamine alone and in rats receiving high dose melamine and NaCl respectively, developed papillomas. Papillomas developed in rats receiving lower dose melamine alone. The occurrence of tumors correlated with calculus (melamine uric acid salt) formation and papillomatosis. Male and female Fisher 344 rats and B6C3F1 mice were fed melamine in the diet for 103 weeks. Twenty percent of the males at high dose and only 2% at the low dose and none of the controls had bladder stones. Seven of the eight urinary bladders with transitional cell carcinomas and three of the remaining 41 bladders without neoplasms had stones. There was statistically significant correlation between the bladder stones and bladder tumors. Fifty percent of a single oral dose of melamine was recovered in the urine of rats within 6 hr. After administration of a single dose of (14)-C-melamine to adult Fisher 344/N rats, 90% of the dose was excreted in the urine within the first 24 hr. Most of the radiolabel was concentrated in the kidney and bladder and negligible amounts were detected in exhaled air and feces. The radiolabelled material found in the plasma and urine indicated that melamine was not metabolized in rats. Melamine induced lambda prophage in Escherichia coli (WP2s-lambda) but did not induce reverse mutation in Salmonella typhimurium in presence or absence of an exogenous metabolic activation system . Sex linked recessive lethal mutations were not induced in Drosophila melanogaster. Melamine did not induce gene linked mutation in Salmonella typhimurium or sister chromatid exchange in Chinese hamster cells in vitro or micronuclei in mouse bone marrow in vivo. Melamine by oral administration has produced urinary bladder and ureteral carcinomas in male rats but only urinary hyperplasia in male mice. The occurrence of urinary tumors in male rats correlated strictly with calculus formation and exposure to higher doses. The dose dependence was confirmed by other studies in male rats in which concomitant administration of sodium chloride to increase urinary output resulted in a decreased tumor yield. Melamine causes carcinomas of the urinary bladder at high doses (in male rats). Formation of bladder stones occurred and these calculi are necessary for the induction of tumours. Carcinomas are induced by continuous irritation of the bladder epithelium by the calculi, so that melamine acts only indirectly as a non-genotoxic carcinogen. (L1777) Toxicity Data LC50 (rat) = 3,248 mg/m3 LD50: 3161 mg/kg (Oral, Rat) (L1777) LD50: 3296 mg/kg (Oral, Mouse) (L1777) LD50: > 1000 mg/kg (Dermal, Rabbit) (L1777) LC50: 3248 mg/m3 (Inhalation, Rat) (L1777) Interactions The major pet food recall associated with acute renal failure in dogs and cats focused initially on melamine as the suspect toxicant. In the course of the investigation, cyanuric acid was identified in addition to melamine in the offending food. The purpose of this study was to characterize the toxicity potential of melamine, cyanuric acid, and a combination of melamine and cyanuric acid in cats. In this pilot study, melamine was added to the diet of 2 cats at 0.5% and 1%, respectively. Cyanuric acid was added to the diet of 1 cat at increasing doses of 0.2%, 0.5%, and 1% over the course of 10 days. Melamine and cyanuric acid were administered together at 0%, 0.2%, 0.5%, and 1% to 1 cat per dose group. No effect on renal function was observed in cats fed with melamine or cyanuric acid alone. Cats dosed with a combination were euthanized at 48 hours after dosing because of acute renal failure. Urine and touch impressions of kidneys from all cats dosed with the combination revealed the presence of fan-shaped, birefringent crystals. Histopathologic findings were limited to the kidneys and included crystals primarily within tubules of the distal nephron, severe renal interstitial edema, and hemorrhage at the corticomedullary junction. The kidneys contained estimated melamine concentrations of 496 to 734 mg/kg wet weight and estimated cyanuric acid concentrations of 487 to 690 mg/kg wet weight. The results demonstrate that the combination of melamine and cyanuric acid is responsible for acute renal failure in cats. Non-Human Toxicity Values LD50 Mouse ip 112 mg/kg bw LD50 Rabbit dermal >1000 mg/kg bw LD50 Mouse (male) gavage 3.3 g/kg LD50 Mouse (female) gavage 7.0 g/kg For more Non-Human Toxicity Values (Complete) data for MELAMINE (9 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
Melamine appears as colorless to white monoclinic crystals or prisms or white powder. Sublimes when gently heated. (NTP, 1992)
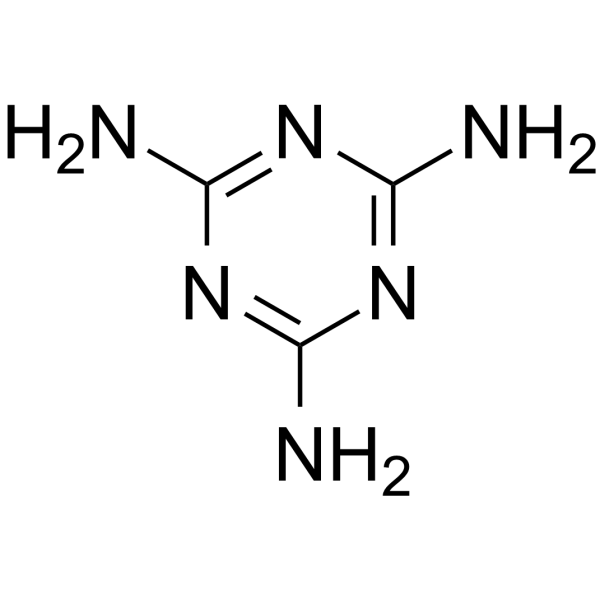
Melamine is a trimer of cyanamide, with a 1,3,5-triazine skeleton. It has a role as a xenobiotic metabolite. It is functionally related to a cyanamide. It is a conjugate base of a melamine(1+). Melamine has been reported in Apis cerana, Euglena gracilis, and Aeromonas veronii with data available. Melamine is an organic base and a trimer of cyanamide, with a 1,3,5-triazine skeleton. Like cyanamide, it contains 66% nitrogen by mass and, if mixed with resins, has fire retardant properties due to its release of nitrogen gas when burned or charred, and has several other industrial uses. Melamine is also a metabolite of cyromazine, a pesticide. It is formed in the body of mammals who have ingested cyromazine. It has been reported that cyromazine can also be converted to melamine in plants. Melamine is described as Harmful if swallowed, inhaled or absorbed through the skin. Chronic exposure may cause cancer or reproductive damage. Eye, skin and respiratory irritant. However, the short-term lethal dose is on a par with common table salt with an LD50 of more than 3 grams per kilogram of bodyweight.[15] U.S. Food and Drug Administration (FDA) scientists explained that when melamine and cyanuric acid are absorbed into the bloodstream, they concentrate and interact in the urine-filled renal tubules, then crystallize and form large numbers of round, yellow crystals, which in turn block and damage the renal cells that line the tubes, causing the kidneys to malfunction. |
| 分子式 |
C3H6N6
|
|---|---|
| 分子量 |
126.12
|
| 精确质量 |
126.065
|
| CAS号 |
108-78-1
|
| 相关CAS号 |
Melamine-15N3;287476-11-3;Melamine-13C3;1173022-88-2;Melamine-15N3,13C3;1246816-14-7
|
| PubChem CID |
7955
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.7±0.1 g/cm3
|
| 沸点 |
557.5±33.0 °C at 760 mmHg
|
| 熔点 |
354 °C
|
| 闪点 |
325.3±12.6 °C
|
| 蒸汽压 |
0.0±1.5 mmHg at 25°C
|
| 折射率 |
1.826
|
| LogP |
-1.37
|
| tPSA |
116.73
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
0
|
| 重原子数目 |
9
|
| 分子复杂度/Complexity |
63.3
|
| 定义原子立体中心数目 |
0
|
| SMILES |
N1C(N([H])[H])=NC(N([H])[H])=NC=1N([H])[H]
|
| InChi Key |
JDSHMPZPIAZGSV-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C3H6N6/c4-1-7-2(5)9-3(6)8-1/h(H6,4,5,6,7,8,9)
|
| 化学名 |
1,3,5-triazine-2,4,6-triamine
|
| 别名 |
Melamine NSC-2130 NSC2130NSC 2130
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~12.5 mg/mL (~99.11 mM)
H2O : ~1 mg/mL (~7.93 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1.25 mg/mL (9.91 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 12.5 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1.25 mg/mL (9.91 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 12.5 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1.25 mg/mL (9.91 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 7.9290 mL | 39.6448 mL | 79.2896 mL | |
| 5 mM | 1.5858 mL | 7.9290 mL | 15.8579 mL | |
| 10 mM | 0.7929 mL | 3.9645 mL | 7.9290 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02719262 | COMPLETED | Device: installing ventilation in the workplace | Environmental Exposure | Kaohsiung Medical University Chung-Ho Memorial Hospital | 2016-03-26 | Not Applicable |
| NCT02251405 | COMPLETED | Behavioral: One bag, stainless container | Healthy Subjects | Ming-Tsang Wu | 2014-10 | Not Applicable |
| NCT00895765 | UNKNOWN STATUS | Calculus | Zhejiang University | 2008-09 | ||
| NCT02724722 | COMPLETED | Behavioral: Assigned intervention Behavioral: No intervention |
Healthy Children and Their Main-care Giver | Kaohsiung Medical University Chung-Ho Memorial Hospital | 2015-03 | Not Applicable |