| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
烟草中的一种系统获得性抗性信号是水杨酸甲酯。它 (0–1 mg/L) 具有水杨酸结合蛋白 2 (SABP2) 的酯酶活性,这是全身组织感知 SAR 信号并将 MeSA 转变为水杨酸 (SA) 所必需的 [2]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Approximately 12-20% of topically applied methyl salicylate may be systemically absorbed through intact skin within 10 hours of application, and absorption varies with different conditions such as surface area and pH. Dermal bioavailability is in the range of 11.8 – 30.7%. For the assessment of potential oral exposure to salicylates, bioavailability is assumed to be 100%. Excreted by kidneys as free salicylic acid (10%), salicyluric acid (75%), salicylic phenolic (10%) and acyl glucuronide (5%), and gentisic acid (less than 1%). After absorption, methyl salicylate is distributed throughout most body tissues and most transcellular fluids, primarily by pH dependent passive processes. Salicylate is actively transported by a low-capacity, saturable system out of the CSF across the choroid plexus. The drug readily crosses the placental barrier. MAY BE ABSORBED RAPIDLY THROUGH INTACT SKIN. BOWEL ABSORPTION IS SOMEWHAT ERRATIC ... ABSORBED AT LEAST IN PART AS THE INTACT ESTER AND SMALL AMT ARE EVEN EXCRETED AS SUCH BY THE KIDNEYS ... . HUMAN SUBJECTS WERE GIVEN 7 MG/KG OF METHYL SALICYLATE BY MOUTH. AFTER 0.25 HOURS THE BLOOD CONCN WAS 1.28 MG%. AFTER 1.5 HOURS THE BLOOD CONCN WAS 1.33 MG%. /FROM TABLE/ At therapeutic doses, conjugation accounts for most salicylic elimination, whereas renal elimination becomes more important with large or multiple doses. A substantial first-pass effect occurs at therapeutic doses. /Salicylates/ Orally ingested salicylates are absorbed rapidly, partly from the stomach but mostly from the upper small intestine. Appreciable conc are found in plasma in less than 30 min; after a single dose, a peak value is reached in about 2 hr and then gradually declines. /Salicylates/ For more Absorption, Distribution and Excretion (Complete) data for METHYL SALICYLATE (6 total), please visit the HSDB record page. Metabolism / Metabolites Minor metabolism may occur in various tissues but hepatic metabolism constitutes the majority of metabolic processes of absorbed methyl salicylate. It is mainly hydrolyzed to salicylic acid via hepatic esterase enzymes. Conjugation with glycine forms salicyluric acid and conjugation with glucuronic forms ester or acyl and ether or phenolic glucuronide, which are the three main metabolites. ...EVIDENCE THAT CONSIDERABLE HYDROLYSIS OF ESTER OCCURS IN INTESTINAL TRACT... IN SOME SPECIES, SUCH AS RABBIT, MAY BE PARTLY EXCRETED AS SULFATE OR GLUCURONIC ACID CONJUGATE ON THE FREE HYDROXYL GROUP. CONJUGATION APPEARS TO TAKE PLACE BEFORE HYDROLYSIS OF THE METHYL ESTER. For small doses 80% of the hepatic metabolism results from conjugation with glycine to form salicyluric acid and with glucuronic acid to form salicyl acyl and phenolic glucuronide. The two parallel pathways (glycine, glucuronide conjugation) have limited capacity and saturate easily above therapeutic doses. /Salicylates/ The biotransformation of salicylates takes place in many tissues, but particularly in the hepatic endoplasmic reticulum and mitochondria. The three chief metabolic products are salicyluric acid (the glycine conjugate), the ether or phenolic glucuronide, and the ester or acyl glucuronide. In addition, a small fraction is oxidized to gentisic acid (2,5-dihydroxybenzoic acid) and to 2,3-dihydroxybenzoic and 2,3,5-trihydroxybenzoic acids; gentisuric acid, the glycine conjugate of gentisic acid, is also formed. /Salicylates/ Biological Half-Life The plasma half-life for salicylate is 2 to 3 hr in low doses and about 12 hr at usual anti-inflammatory doses. The half-life of salicylate may be as long as 15 to 30 hr at high therapeutic doses or when there is intoxication. The plasma half-life for ... salicylate is 2 to 3 hr in low doses and about 12 hr at usual antiinflammatory doses. The half-life of salicylate may be as long as 15 to 30 hr at high therapeutic doses or when there is intoxication. /Salicylates/ |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Degree of albumin binding depends on the plasma concentration of the compound Interactions ... The prolonged and excessive ingestion of analgesic mixtures containing salicylates in combination with acetaminophen or salicylamide can produce papillary necrosis and interstitial nephritis. /Salicylates/ Non-Human Toxicity Values LD50 Rat oral 0.887 g/kg LD50 Rabbit oral 2.8 g/kg LD50 Guinea pig oral 1.060 g/kg LD50 Dog oral 2.1 g/kg LD50 Guinea pig dermal 0.70 ml/kg |
| 参考文献 | |
| 其他信息 |
Methyl salicylate appears as colorless yellowish or reddish liquid with odor of wintergreen. (USCG, 1999)
Methyl salicylate is a benzoate ester that is the methyl ester of salicylic acid. It has a role as a flavouring agent, a metabolite and an insect attractant. It is a benzoate ester, a member of salicylates and a methyl ester. It is functionally related to a salicylic acid. Methyl salicylate (oil of wintergreen or wintergreen oil) is an organic ester naturally produced by many species of plants, particularly wintergreens. The compound was first extracted and isolated from plant species Gaultheria procumbens in 1843. It can be manufactured synthetically and it used as a fragrance, in foods, beverages, and liniments. It forms a colorless to yellow or reddish liquid and exhibits a characteristic odor and taste of wintergreen. For acute joint and muscular pain, methyl salicylate is used as a rubefacient and analgesic in deep heating liniments. It is used as a flavoring agent in chewing gums and mints in small concentrations and added as antiseptic in mouthwash solutions. Methyl Salicylate has been reported in Camellia sinensis, Phellinus tremulae, and other organisms with data available. Methyl 2-hydroxybenzoate is found in beverages. Methyl 2-hydroxybenzoate is present in white wine, tea, porcini mushroom Boletus edulis, Bourbon vanilla, clary sage, red sage and fruits including cherry, apple, raspberry, papaya and plum. Methyl 2-hydroxybenzoate is found in leaves of Gaultheria procumbens (wintergreen). Methyl 2-hydroxybenzoate is a flavouring agent. Methyl 2-hydroxy benzoate is a metabolite found in or produced by Saccharomyces cerevisiae. See also: Salicylic Acid (has active moiety); Clove Oil (part of); Levomenthol; methyl salicylate (component of) ... View More ... Drug Indication Ointments or liniments containing methyl salicylate are applied topically as counter irritant for relief of acute pain associated with lumbago,sciatica and rheumatic conditions. Local analgesics for human and veterinary medicine. Mechanism of Action Counter-irritation is thought to be effective at alleviating musculoskeletal pain as the irritation of the sensory nerve endings is thought to alter or offset pain in the underlying muscle or joints that are served by the same nerves. This is thought to mask the underlying musculoskeletal pain and discomfort. When applied topically, methyl salicylate is thought to penetrate the skin and underlying tissues where it reversibly inhibits cyclooxygenase enzyme and locally and peripherally prevents the production of inflammatory mediators such as prostaglandin and thromboxane A2. Therapeutic Uses OINTMENTS OR LINIMENTS CONTAINING METHYL SALICYLATE ARE APPLIED TOPICALLY AS COUNTERIRRITANTS FOR RELIEF OF PAIN ASSOCIATED WITH LUMBAGO, SCIATICA, AND RHEUMATIC CONDITIONS. FORMERLY USED INTERNALLY IN SMALL DOSES AS A CARMINATIVE. MEDICATION (VET): ORALLY, PRIMARILY AS FLAVORING AGENT OR AS CARMINATIVE; TOPICALLY, AS IRRITANT OR COUNTERIRRITANT AIDED BY MASSAGE OR RUBBING AS IN UDDER OINTMENTS (1-3% CONCN), POULTICES & COUNTERIRRITANT MIXT (@ LEAST 5-10%) OVER SORE JOINT, MUSCLE, & BONE AREAS. LOCAL ANALGESIC FOR HUMAN AND VETERINARY MEDICINE Drug Warnings OINTMENTS OR LINIMENTS ... . SHOULD NOT BE APPLIED TO BURNED AREAS OR TO OTHERWISE DAMAGED SKIN...USUALLY IN CONCN FROM 10-25% ... . ABSORPTION OF METHYL SALICYLATE CAN OCCUR THROUGH THE SKIN, & DEATH HAS RESULTED FROM SYSTEMIC POISONING FROM THE LOCAL MISAPPLICATION OF THE DRUG. IT IS A COMMON PEDIATRIC POISON, & ITS USE SHOULD BE STRONGLY DISCOURAGED. Children with fever and dehydration are particularly prone to intoxication from relatively small doses of salicylate. ... The use of aspirin is contraindicated in children and adolescents with febrile viral illnesses because of the risk of Reye's syndrome. /Salicylates/ Pharmacodynamics Methyl salicylate relieve musculoskeletal pain in the muscles, joints, and tendons by causing irritation and reddening of the skin due to dilated capillaries and increased blood flow. It is pharmacologically similar to aspirin and other NSAIDs but as a topical agent it primarily acts as a rubefacient and skin irritant. Counter-irritation is believed to cause a soothing sensation of warmth. |
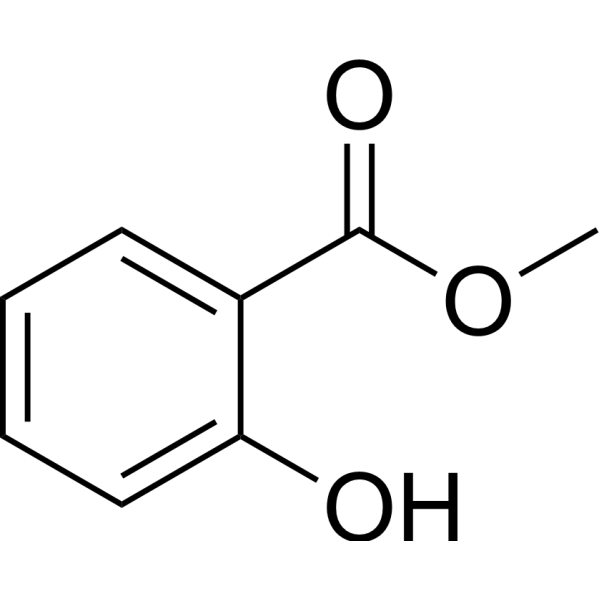
| 分子式 |
C8H8O3
|
|---|---|
| 分子量 |
152.14
|
| 精确质量 |
152.047
|
| CAS号 |
119-36-8
|
| 相关CAS号 |
Methyl Salicylate-d4;1219802-12-6
|
| PubChem CID |
4133
|
| 外观&性状 |
Colorless to light yellow liquid
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
222.0±0.0 °C at 760 mmHg
|
| 熔点 |
-8 °C
|
| 闪点 |
86.8±12.6 °C
|
| 蒸汽压 |
0.1±0.4 mmHg at 25°C
|
| 折射率 |
1.547
|
| LogP |
2.23
|
| tPSA |
46.53
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
11
|
| 分子复杂度/Complexity |
144
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O(C([H])([H])[H])C(C1=C([H])C([H])=C([H])C([H])=C1O[H])=O
|
| InChi Key |
OSWPMRLSEDHDFF-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C8H8O3/c1-11-8(10)6-4-2-3-5-7(6)9/h2-5,9H,1H3
|
| 化学名 |
methyl 2-hydroxybenzoate
|
| 别名 |
Gaultheria oil; Betula; Methyl salicylate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
Ethanol : ~25 mg/mL (~164.31 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (16.43 mM) (饱和度未知) in 10% EtOH + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 EtOH 储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL 生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (16.43 mM) (饱和度未知) in 10% EtOH + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清乙醇储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.5729 mL | 32.8645 mL | 65.7289 mL | |
| 5 mM | 1.3146 mL | 6.5729 mL | 13.1458 mL | |
| 10 mM | 0.6573 mL | 3.2864 mL | 6.5729 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。