| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg | |||
| 250mg | |||
| 500mg | |||
| Other Sizes |
| 靶点 |
Leishmania; Akt1 E17K mutant; Akt1 (IC50 = 2.7 nM); Akt3 (IC50 = 8.1 nM); Akt2 (IC50 = 174 nM)
|
|---|---|
| 体外研究 (In Vitro) |
Miransertib(ARQ-092;化合物 21a)在具有 PIK3CA/PIK3R1 突变的细胞系中表现出比在具有野生型 (wt) PIK3CA/PIK3R1 或 PTEN 缺失的细胞系中更强的抗增殖活性,这些细胞系来自多种来源的细胞系。肿瘤类型。在 AN3CA 和 A2780 细胞中,miransertib 对 p-Akt (S473) 和 p-Akt (T308) 表现出优异的抑制作用。使用 Miransertib (IC50=0.31 M),下游蛋白 p-PRAS40 (T246) 被抑制[1]。 Miransertib 对杜氏乳杆菌或亚马逊乳杆菌感染的巨噬细胞的细胞内无鞭毛体显着有效。此外,在利什曼原虫感染的巨噬细胞中,miransertib 增加了 mTOR 依赖性自噬。 [2]
|
| 体内研究 (In Vivo) |
在大鼠 (5 mg/kg) 和猴子 (10 mg/kg) 中,miransertib(ARQ-092;化合物 21a)表现出良好的绝对口服生物利用度,F 值分别为 62% 和 49%。大鼠的 t1/2 值为 17 小时,而猴子为 7 小时,表明大鼠的半衰期比猴子更长。大鼠和猴子的 Cmax 值分别为 198 ng/mL 和 258 ng/mL,AUCinf 值分别为 5496 hng/mL 和 2960 hng/mL[1]。 Miransertib(ARQ-092;化合物 21a)可抑制人类异种移植小鼠子宫内膜腺癌模型中的肿瘤生长[1]。
|
| 酶活实验 |
Miransertib (ARQ-092) 是一种口服生物可利用的、选择性的、有效的变构 Aktin 抑制剂,对 Akt1、Akt2、Akt3 的 IC50 值分别为 2.7 nM、14 nM 和 8.1 nM。
AKT1(1–480)、AKT2(1–481)和AKT3(1–479)阿尔法筛查分析[4] 使用GSK3衍生的生物素化肽底物、crosstide(生物素GRPRTSSFAEG)和AlphaScreen(扩增发光邻近均匀测定)技术测定AKT活性。在10%DMSO中以所需终浓度的10倍制备试验化合物和对照,并将每种化合物2.5μL加入反应板(Corning 96孔半面积固体白色非结合表面板)的每个孔中。将全长未磷酸化的AKT在测定缓冲液(50 mM Tris,pH 8.0,0.02 mg/mL BSA,10 mM MgCl2,1 mM EGTA,10%甘油,0.2 mM Na3VO4,1 mM DTT,0.1 mMβ-甘油磷酸盐和0.2 mM NaF)中稀释,并以17.5μL的体积加入每个孔中,使25μL反应中的终浓度为8 nM(AKT1)、63 nM(AK T2)或13 nM(AKT3)。在室温下预孵育20分钟后,通过加入5μL在测定缓冲液中稀释的活化混合物来启动激酶反应,该混合物含有生物素化的交叉肽、PDK1、MAPKAPK2、DOPS/DOPC、PtdIns(3,4,5)P3和ATP,最终浓度为60 nM生物素化交叉肽、0.1 nM(AKT1、AKT3)或0.3 nM M(AKT1、AKT2)或18μM(AKT3)ATP。将平板在室温下孵育30分钟,通过加入10μL在测定缓冲液中制备的停止/检测混合物在黑暗中停止反应,该混合物含有EDTA、AlphaScreen链霉抗生物素供体和蛋白A受体珠,以及终浓度为10mM EDTA的磷酸化AKT底物抗体,500 ng/孔的AlphaScreen链霉素供体珠和蛋白A接收器珠,以及最终稀释度为1:350的磷酸化AK T底物抗体。在室温下在黑暗中孵育测定板90分钟,并在PerkinElmer Envision多标记板阅读器上读取板(激发波长,640 nm;发射波长,570 nm)。所有报告的IC50值均为至少n=2次测定的几何平均值。 CYP450抑制试验[4] 将人肝微粒体(0.25 mg/mL)、探针底物[3μM咪达唑仑(3A4)、5μM丁咯洛尔(2D6)、100μM甲磺丁脲(2C9)、10μM紫杉醇(2C8)、80μM S-美芬妥英(2C19)和50μM非那西丁(1A2)]、3.3 mM MgCl2、1 mM NADPH和溶解在DMSO(0.1%终浓度)中的化合物(0.1-10μM)孵育10分钟,然后使用含利血平(内标)的乙腈停止反应。在35°C的恒定氮气流下蒸发溶剂,将所得化合物溶解在100μL水中进行LC/MS/MS分析。使用由0.5μM酮康唑(3A4)、1μM磺胺苯唑(2C9)、1µM奎尼丁(2D6)、2μM槲皮素(2C8)、1微米诺卡酮(2C19)和0.5μMα-萘黄酮(1A2)组成的抑制剂混合物作为阳性对照。仅含有DMSO(无化合物)的培养基作为100%活性对照。基于100%活性对照的信号计算抑制百分比,并使用XLfit4中的拟合模型205(单部位剂量反应)估算或确定IC50值。 跨物种NADPH依赖性微粒体稳定性测定[4] 将人、CD-1小鼠和比格犬肝微粒体(0.25 mg/mL)、3.3 mM MgCl2和NADPH再生系统(0.4单位/mL G6PDH、1.3 mM NADP+和3.3 mM G6P)与1μM化合物一起孵育0、3、6、10、15或30分钟。用含华法林的乙腈(内标)停止孵育,并通过LC/MS/MS分析样品。峰面积比用于确定每个时间点与时间零点相比的剩余百分比。使用单指数衰变方程估算半衰期值。咪达唑仑和普萘洛尔(仅雌性小鼠)用作阳性对照。 |
| 细胞实验 |
将细胞(MDA-MB-453:1.5 106;NCI-H1650:1 106;KU-19-19:0.7 106)接种到 6 孔板中,孵育过夜,然后暴露于含有不同浓度AKT 抑制剂(ARQ 092、ARQ 751、MK-2206、GDC-0068)2 小时。在预定条件下处理细胞后获得裂解物。 SDS-PAGE 后,使用免疫印迹从提取物中分离蛋白质。
|
| 动物实验 |
Male SCD mice
100 mg/10ml/kg oral administration |
| 药代性质 (ADME/PK) |
Compounds 9a,b, 21a–c, and 23 did not significantly inhibit CYP450 1A2, 2C8, 2D6, and 3A4. Compounds 9a and 9b inhibited CYP450 2C9 with a submicromolar IC50 value, while 9a also inhibited CYP450 2C19 (IC50 ≤ 1 μM). All compounds were adequately stable in liver microsomes (human, mouse, and dog). In general, we found that compounds from this chemical series possess adequate in vitro ADME properties, and compound ARQ 092 (21a) in particular showed good biochemical inhibition, cellular knockdown of AKT phosphorylation, and ADME properties. In a mouse pharmacokinetic study (po at 100 mg/kg, iv at 5 mg/kg), compound ARQ 092 (21a) showed an oral bioavailability of 23%. An in vivo pharmacodynamic assessment of ARQ 092 (21a) using NCr-M nude mice implanted with AN3CA tumor xenografts was reported in our recent publication. Compound 21a resulted in 99%, 95%, and 58% reductions in p-AKT (S473), p-AKT (T306), and p-PRAS40 (T246), respectively, after tumor-bearing mice were treated with 100 mg/kg po (Table 8). The inhibition of phosphorylation was sustained at 8 h. The plasma concentration of compound 21a at 1 h was 2.1 μM and decreased to 0.26 μM at 8 h, while in the tumor, the concentration was 21.0 μM at 1 h and 9.6 μM at 8 h. The concentrations in the tumor tissues were significantly higher than in the plasma, indicating a marked preference for tissue accumulation compared with the vasculature compartment.[1]
Definitive single dose pharmacokinetic studies were conducted in rats and monkeys (Table 9). ARQ 092 (21a) showed good absolute oral bioavailability in rats and monkeys with F values of 62% and 49%, respectively. The compound was more slowly absorbed in rats compared to monkeys with Tmax values of 8.0 h for rats versus 4.3 h for monkeys. The half-life was also longer in rats compared to monkeys with t1/2 values of 17 h in rats versus 7 h in monkeys. The Cmax was 198 and 258 ng/mL and the AUCinf was 5496 and 2960 h·ng/mL in rats and monkeys, respectively. |
| 参考文献 | |
| 其他信息 |
Miransertib is under investigation in clinical trial NCT01473095 (Phase 1 Dose Escalation Study of ARQ 092 in Adult Subjects With Advanced Solid Tumors and Recurrent Malignant Lymphoma).
Miransertib is an orally bioavailable inhibitor of the serine/threonine protein kinase AKT (protein kinase B) with potential antineoplastic activity. Miransertib binds to and inhibits the activity of AKT in a non-ATP competitive manner, which may result in the inhibition of the PI3K/AKT signaling pathway. This may lead to the reduction in tumor cell proliferation and the induction of tumor cell apoptosis. The AKT signaling pathway is often deregulated in cancer and is associated with tumor cell proliferation, survival and migration. The work in this paper describes the optimization of the 3-(3-phenyl-3H-imidazo[4,5-b]pyridin-2-yl)pyridin-2-amine chemical series as potent, selective allosteric inhibitors of AKT kinases, leading to the discovery of ARQ 092 (21a). The cocrystal structure of compound 21a bound to full-length AKT1 confirmed the allosteric mode of inhibition of this chemical class and the role of the cyclobutylamine moiety. Compound 21a demonstrated high enzymatic potency against AKT1, AKT2, and AKT3, as well as potent cellular inhibition of AKT activation and the phosphorylation of the downstream target PRAS40. Compound 21a also served as a potent inhibitor of the AKT1-E17K mutant protein and inhibited tumor growth in a human xenograft mouse model of endometrial adenocarcinoma.[1] Leishmaniasis is amongst the most important neglected diseases, afflicting more than 12 million people in 88 countries. There is an urgent need for safe orally bioavailable and cost-effective drugs for the treatment of leishmaniasis. It has recently been shown that Leishmania activates host macrophage serine/threonine kinase Akt, to promote survival of both parasites and infected cells. Here, we sought to evaluate a compound, Miransertib (ARQ 092), an orally bioavailable and selective allosteric Akt inhibitor currently in clinical trials for patients with PI3K/Akt-driven tumors or Proteus syndrome. Miransertib was tested against Leishmania donovani and Leishmania amazonensis, causative agents of visceral and cutaneous leishmaniasis, respectively. Cultured promastigotes were susceptible to Miransertib. In addition, Miransertib was markedly effective against intracellular amastigotes of L. donovani or L. amazonensis-infected macrophages. Miransertib also enhanced mTOR dependent autophagy in Leishmania-infected macrophages, which may represent one mechanism of Miransertib-mediated killing of intracellular Leishmania. Whereas parasite clearance in the spleen of mice infected with L. donovani and treated with Miransertib was comparable to that when treated with miltefosine, Miransertib caused a greater reduction in the parasite load in the liver. In the cutaneous leishmaniasis infection model, lesions were reduced by 40% as compared to mock treated mice. Together, these results provide direct evidence to support the conclusion that Miransertib is an excellent lead compound for the development of a new oral drug therapy for visceral and cutaneous leishmaniasis.[2] |
| 分子式 |
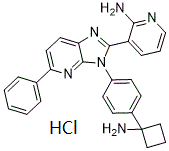
C27H25CLN6
|
|---|---|
| 分子量 |
468.99
|
| 精确质量 |
468.182
|
| 元素分析 |
C, 69.15; H, 5.37; Cl, 7.56; N, 17.92
|
| CAS号 |
1313883-00-9
|
| 相关CAS号 |
Miransertib;1313881-70-7
|
| PubChem CID |
67305743
|
| 外观&性状 |
Light yellow to brown solid powder
|
| tPSA |
95.6
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
34
|
| 分子复杂度/Complexity |
653
|
| 定义原子立体中心数目 |
0
|
| SMILES |
Cl.NC1(C2C=CC(=CC=2)N2C(C3=CC=CN=C3N)=NC3C=CC(C4C=CC=CC=4)=NC2=3)CCC1
|
| InChi Key |
DRHSWSSVIKDJME-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C27H24N6.ClH/c28-24-21(8-4-17-30-24)25-32-23-14-13-22(18-6-2-1-3-7-18)31-26(23)33(25)20-11-9-19(10-12-20)27(29)15-5-16-27;/h1-4,6-14,17H,5,15-16,29H2,(H2,28,30);1H
|
| 化学名 |
3-[3-[4-(1-aminocyclobutyl)phenyl]-5-phenylimidazo[4,5-b]pyridin-2-yl]pyridin-2-amine;hydrochloride
|
| 别名 |
ARQ092 hydrochloride; MK-7075; ARQ-092 hydrochloride; MK7075; Miransertib HCl; Miransertib (ARQ 092) HCl; Miransertib hydrochloride; Miransertib (hydrochloride); CHEMBL4523032; 3-(3-(4-(1-aminocyclobutyl)phenyl)-5-phenyl-3H-imidazo[4,5-b]pyridin-2-yl)pyridin-2-amine hydrochloride; ARQ 092
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.33 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1322 mL | 10.6612 mL | 21.3224 mL | |
| 5 mM | 0.4264 mL | 2.1322 mL | 4.2645 mL | |
| 10 mM | 0.2132 mL | 1.0661 mL | 2.1322 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Status | Interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04980872 | Active Recruiting |
Drug: Miransertib | PIK3CA-Related Overgrowth Spectrum (PROS) Proteus Syndrome (PS) |
Merck Sharp & Dohme LLC | November 2, 2021 | Phase 2 |
| NCT01473095 | Completed | Drug: ARQ 092 | Solid Tumor Malignant Lymphoma |
ArQule, Inc. | November 2011 | Phase 1 |
| NCT02594215 | Completed | Drug: MK-7075 (miransertib) | Proteus Syndrome | National Human Genome Research Institute | November 16, 2015 | Phase 1 |