| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
Choline kinase α (CHKα)
|
|---|---|
| 体外研究 (In Vitro) |
MN58b 对亲本 Suit2 007 细胞的 IC50 为 3.14 μM,对吉西他滨缀合细胞的 IC50 为 0.77 μM [1]。在 1 μM 和 5 μM 生长条件下,MN58b(1-5 μM;72 小时;SK-PC-1、Suit2 008、IMIM-PC2 和 RWP-1 细胞)显着影响所有细胞系的集落形成[1]。 SK-PC-1,Suit2 008,IMIM; MN58b((1-10 μM;24-48 小时)。
CHKαIMN58b的抗增殖活性与CHKα表达水平有关[1] MN58b选择性抑制CHKα;因此,我们观察到IMIM-PC-2细胞中胆碱合成磷酸胆碱的减少(图4A)。我们分析了MN58b对四种PDAC细胞系(SK-PC-1、Suit2008、IMIM-PC-2和RWP-1)生长的影响。MN58b在1μmol/L时对集落形成有显著影响,在所有测试的细胞系中,在5μmol/L时生长完全被抑制(图4B)。在12个PDAC细胞系中,MN58b的IC50范围为0.23至3.2μmol/L。我们发现CHKα蛋白表达与MN58b敏感性之间存在直接关系(R2=0.88;图4B和补充表S3)。Suit2 028和Suit2 007细胞中CHKα的敲除与IC50的增加有关(补充表S4)。 为了确定MN58b的作用机制,我们用浓度递增的MN58b(1-10μmol/L)处理PDAC细胞24或48小时,并通过Annexin V分析凋亡。CHKα表达与48小时AnnexinⅤ阳性细胞的百分比之间存在直接相关性(图4C)。通过蛋白质印迹分析切割的胱天蛋白酶-9证实了凋亡的诱导;观察到剂量-反应关系(数据未显示)。因此,MN58b诱导细胞凋亡,这种反应与CHKα表达相关。这些结果表明,CHKα可能是MN58b反应的预测标志物。 MN58b和化疗药物的联合作用[1] 原发性和获得性耐药性都导致吉西他滨治疗PDAC的疗效有限。我们使用亲代和吉西他滨耐药的Suit2007细胞来评估耐药性与MN58b敏感性之间的关系。MN58b对亲代细胞和耐药细胞的IC50分别为3.14μmol/L和0.77μmol/L,支持MN58b可能是吉西他滨耐药肿瘤的治疗替代品的观点。 为了测试MN58b与PDAC治疗中其他化疗药物的协同作用(5),我们用吉西他滨、奥沙利铂或5-FU加MN58b以低于IC50的浓度处理表达不同水平CHKα的PDAC细胞(SK-PC-1、Suit2028和RWP-1)。协同作用以CI表示。在Suite2 028细胞中,所测试的组合均未显示出增加的效果。在另外两种细胞系中,MN58b与吉西他滨和5-FU联合使用显示出相加效应,与奥沙利铂联合使用显示协同效应(SK-PC-1,CI=0.23;RWP-1,CI=0.39;图4D和补充图S4)。这些发现支持MN58b与其他化疗药物联合使用。 对MN58b的抗性是由ABCB转运蛋白1和4[1]的上调介导的 为了评估对CHKI产生耐药性的机制,我们通过不断增加药物浓度的连续培养,从亲本IMIM-PC-2细胞中产生了一个MN58b抗性系。治疗9个月后,建立IMIM-PC-2-R细胞;其IC50为156μmol/L,约为亲本细胞的30倍。IMIM-PC-2-R细胞的集落形成能力不受10μmol/L MN58b处理的影响(图5A)。IMIM-PC-2-R显示出比亲本细胞更低的基线增殖率(图5A),以及胆碱摄取减少(约50%;图5B)。然而,CHKα酶活性在抗性细胞和亲本细胞中相似(图5C)。 MN58b治疗的细胞效应。[2] MDA-MB-231和HT29细胞分别用MN58b处理4、13、19、30和48小时(MDA-MB-231:48小时(HT29),MN58b的药理学活性浓度对应于磺基罗丹明B测定96小时(分别为6和2.5μmol/L)获得的5×IC50。孵育48小时后,每个培养瓶中经MN58b处理的细胞数量占对照组的百分比在MDA-MB-231(图1A)和HT29中分别显著减少至78±10%(P<0.04)和48±5%(P<0.01),这与增殖减少一致。相比之下,在用非活性类似物ACG20b治疗后,MDA-MB-231和HT29的细胞数量分别与对照组相似(95±8%,P=0.5)和(95±7%,P=0.4)。为了进一步表征MN58b的细胞效应,通过流式细胞术测定了MN58b处理后附着细胞的细胞周期分布。在用MN58b处理长达30小时后,MDA-MB-231细胞的细胞周期分布没有统计学上的显著影响。然而,在48小时时,G1期细胞的百分比从43±4%显著增加到54±3%(P=0.01),而S期和G2期细胞的比例分别从43±3%显著降低到35±4%(P=0.02)和从14±1%显著降低到11±1%(P=0.001)。对于HT29细胞,用MN58b处理48小时也导致G1期细胞百分比从60±4%显著增加到80±2%(P=0.007),S期和G2期细胞百分比分别从32±4%显著降低到17±3%(P=0.02)和从8±2%显著降低到3±1%(P=0.04)。另一方面,用非活性类似物ACG20b治疗48小时对MDA-MB-231和HT29细胞的细胞周期分布没有统计学上的显著影响(P=0.4;数据未显示)。 细胞提取物的体外1H-MRS和31P-MRS。[2] 使用体外用MN58b处理的癌细胞的MRS来鉴定胆碱激酶抑制的潜在非侵入性标志物。图1B显示了对照和48小时MN58b处理的MDA-MB-231细胞的31P-MR光谱。对对照组和MN58b处理的MDA-MB-231细胞在4、13、16、30和48小时的31P-MR光谱的详细分析表明,MN58b治疗导致磷酸胆碱水平相对于对照组出现统计学上显著的时间依赖性下降,最早在4小时开始(81±6%,P=0.04),相对于对照组,在48小时降至40±2%(P=0.00001)(图1A)。HT29细胞与MN58b孵育48小时后,磷酸胆碱相对于对照组也出现了统计学上的显著下降(42±13%,P=0.03)(图1C)。其他31P-MR可检测代谢物(甘油磷酸乙醇胺、甘油磷酸胆碱和核苷酸三磷酸)的变化在两种细胞系中均无统计学意义(P>0.05)。由于两种细胞系中的磷酸乙醇胺信号非常小,因此无法准确测量细胞提取物中的磷酸酒精胺水平。 还研究了用ACG20b(非活性类似物)处理的MDA-MB-231和HT29细胞的31P-MR光谱,以验证31P-MRS检测到的磷酸胆碱下降是由于MN58b对胆碱激酶的抑制作用。在用ACG20b治疗48小时后,MDA-MB-231(88±11%,P=0.2)或HT29(88±10%,P=0.2)细胞中的磷酸胆碱水平没有观察到统计学上的显著变化(表1;图1B和C)。表1和图1A总结了用MN58b治疗一段时间后MDA-MB-231细胞中磷酸胆碱浓度的变化。 还研究了对照和MN58b处理的MDA-MB-231细胞在4、13、19、30和48小时的提取物的1H-MR光谱。与对照组相比,在与MN58b孵育19小时后,观察到总胆碱含量(胆碱+磷酸胆碱+甘油磷酸胆碱)随时间显著下降(63±17%,P=0.03),在MN58b治疗后48小时降至49±9%(P=0.002)。总胆碱的减少与31P-MRS测量的磷酸胆碱水平的下降相似;因此,总胆碱的减少是由于磷酸胆碱的下降,而细胞内胆碱和甘油磷酸胆碱水平不受MN58b治疗的影响。 磷脂酰胆碱是合成磷脂酰胆碱所必需的。因此,为了确定磷酸胆碱的下降是否会导致磷脂酰胆碱水平的类似下降,测量了对照和经MN58b处理的MDA-MB-231和HT29细胞的脂质组分的1H-MR光谱。用MN58b处理两种细胞系后,未检测到磷脂酰胆碱水平的显著变化[使用在3.32ppm共振的N(CH3)3峰](P>0.4;数据未显示)。这些结果与之前使用NIH3T3细胞和人类原代淋巴细胞的发现一致。 细胞提取物中的胆碱激酶活性及其与MRS测量的磷酸胆碱水平的相关性。[2] 14C-胆碱标记被用作MDA-MB-231细胞在用MN58b进行时间过程处理后胆碱激酶活性的指标(表1;图1A)。发现磷酸胆碱浓度(通过MRS测量)与胆碱激酶活性之间存在显著相关性(r2=0.95,P=0.0008)。 |
| 体内研究 (In Vivo) |
在 HT29 和 MDA-MB-231 异种移植物中,用 MN58b(4 mg/kg;腹腔注射;每天一次;持续 5 天;MF-1 裸鼠)治疗显着减少磷酸单酯。研究发现,磷酸胆碱水平和胆汁。
HT29异种移植物的体内1H-MRS和31P-MRS。[2] 在这项研究中,MN58b抑制了HT29异种移植物中70%的肿瘤生长(治疗组与对照组的百分比,T/C百分比),证实了之前的发现。 图2A至D显示了HT29肿瘤在治疗前MN58b和治疗后的体内1H-MR和31P-MR光谱。在MN58b治疗后,还观察到总胆碱浓度(P=0.01)和磷酸单酯/总磷信号比(P=0.05)的统计显著降低(表2A)。在对照(赋形剂治疗)肿瘤组中没有观察到总胆碱浓度的统计学显著变化。在对照组中观察到磷酸单酯/总磷信号比在统计学上显著增加(P=0.04)。 MDA-MB-231异种移植物的体内31P-MRS。[2] 先前的研究表明,当MDA-MB-231人乳腺癌异种移植物用MN58b治疗时,生长延迟具有统计学意义。与这些结果一致,我们发现MN58b治疗5天可抑制85%(%T/C)的肿瘤生长。 在MN58b治疗后,MDA-MB-231肿瘤的体内31P-MRS显示磷酸单酯/总磷信号比在统计学上显著降低(P=0.05)(表2B)。在对照(载体治疗)肿瘤组中没有观察到统计学上的显著变化。 |
| 酶活实验 |
胆碱激酶活性[1]
将游离的3H-胆碱加入到反应混合物中(MgCl2 10 mmol/L,KCl 100 mmol/L,ATP 500μmol/L,Tris pH 7.5 100 mmol/L),并使用改良的Bligh和Dyer测定法从3H-胆碱转化为3H-磷酸胆碱的量中测定其磷酸化。在加入3H-氯化胆碱之前,被动裂解缓冲液的使用确保了裂解物中的酶活性。通过加入甲醇/氯仿在60分钟时停止反应,以有效地启动脂质提取步骤。然后使用四苯硼酸盐进行相萃取,将胆碱与磷酸胆碱分离,并使用闪烁计数器测定每个部分中3H的量。 细胞提取物中胆碱激酶活性的酶法测定。[2] 在用6μmol/L的MN58b处理4、13、19、30和48小时后,将来自三个p60平板(1×106)的指数生长细胞在80μL的裂解缓冲液[1.5 mmol/L MgCl2、0.2 mmol/L EDTA、0.3 mol/L NaCl、25 mmol/L HEPES(pH 7.5)、20 mmol/Lβ-甘油磷酸和0.1%Triton X-100]中裂解至终浓度为3μg/L。由此,如前所述,在含有100 mmol/L Tris-HCl(pH 8)、100 mmol/L MgCl2和10 mmol/L ATP的缓冲液中,每个点使用90μg总蛋白作为胆碱激酶的来源。 细胞提取物的体外1H-MRS和31P-MRS。[2] 为了获得MR光谱,如前所述,从细胞培养物中提取对数期的1×107至2×107细胞。简而言之,用冰冷的生理盐水冲洗细胞,并用6mL冰冷的甲醇固定。然后将细胞从培养瓶表面刮下,收集到试管中,在室温下涡旋30秒,以优化从破裂细胞中提取磷脂代谢物。然后向每个试管中加入氯仿(6mL),然后加入等体积的去离子水。相分离后,使用冷冻干燥机去除上层甲醇/水相中的溶剂,通过将氯仿蒸发至干燥来回收脂质代谢物,并将样品储存在-80°C下直至分析。在获取MRS光谱之前,将水溶性代谢物重新悬浮在氧化氘(D2O)中用于1H-MRS,或将D2O与10 mmol/L EDTA(pH 8.2)重新悬浮在31P-MRS中用于31P-MRS,将脂质代谢产物重新悬浮在氘代氯仿中用于1H-MS。在室温下,在500 MHz Bruker光谱仪上使用30度翻转角、1秒弛豫延迟、100ppm的光谱宽度和31P的32 K数据点获取1H-MRS和1H解耦31P-MRS光谱;30度翻转角、1秒弛豫延迟、12ppm的光谱宽度和1H(脂质)的32K数据点;以及90度翻转角、1秒弛豫延迟、12ppm的光谱宽度、64K数据点和1H(水溶液)预饱和的HDO共振抑制。代谢物含量通过积分确定,并相对于内部参考的峰值积分进行归一化[1H-MRS为0.15%3-(三甲基甲硅烷基)丙酸-2,2,3,3-d4酸钠盐(水组分)或0.03%四甲基硅烷(脂质组分),31P-MRS为亚甲基二膦酸(70μL,2 mmol/L)],并校正信号强度饱和度和每个样品提取的细胞数量。 肿瘤提取物的体外31P-MRS。[2] 如前所述,冷冻夹持的肿瘤在6%高氯酸中提取。将中和的提取物冷冻干燥并在1mL D2O中复溶,将提取物(0.5mL)置于5-mm NMR管中。对于1H-MRS,通过使用以水频率为中心的门控照射来抑制水共振。向样品中加入3-(三甲基甲硅烷基)丙酸-2,2,3,3-d4钠盐(50μL,5mmol/L)进行化学位移校准和定量。在MRS分析之前,用高氯酸或KOH将样品的pH值重新调节至7。对于1H-MRS研究后进行的31P-MRS,向每个样品中加入EDTA(50μL,60 mmol/L)以螯合金属离子,向每个样本中加入亚甲基二膦酸(50μL,5 mmol/L)以进行化学位移校准和定量。在相同条件下获得对照和处理动物的提取光谱。 |
| 细胞实验 |
细胞活力测定[1]
细胞类型: SK-PC-1、Suit2 008、IMIM-PC2 和 RWP-1 - PC2 和 RWP-1 细胞)诱导细胞,这种反应与 CHKα 表达相关[1]。细胞 测试浓度: 1 µM、5 µM 孵育时间:72 小时 实验结果: 抑制细胞生长。 细胞凋亡分析 [1] 细胞类型: SK -PC-1、Suit2 008、IMIM-PC2 和 RWP-1 细胞 测试浓度: 1 µM、2 µM、5 µM、10 µM 孵育时间: 24 小时和 48 小时 实验结果: 诱导细胞凋亡。 产生耐MN58b的细胞系[1] 为了产生MN58b抗性IMIM-PC-2细胞,从0.1μmol/L开始加入MN58b。对照IMIM-PC.2细胞在没有药物的情况下培养。MN58b浓度在每次细胞传代时每周增加50%(融合时分裂1:3);终浓度为8μmol/L。 为了产生对吉西他滨耐药的PDAC细胞系,使用了增量剂量法。起始浓度为35nmol/L 2′-脱氧-2′,2′-二氟胞苷单盐酸盐。吉西他滨浓度保持恒定,每次细胞传代时增加1.5至2倍。最终浓度为250 nmol/L。通过STR分析鉴定抗性细胞。 生长和存活率测定[1] 将细胞(每孔5×104个)接种在6孔板上,一式三份,胰蛋白酶处理并计数。为了确定存活率,将细胞(每孔2×104个)接种在24孔板中。24小时后,移除培养基并加入MN58b;72小时后,用3%甲醛固定细胞,用PBS洗涤两次,用25%甲醇中的0.5%结晶紫孵育;用10%乙酸洗脱结晶紫,测定OD590nm。为了评估集落形成,将细胞(每孔5×104个)接种在6孔板中,24小时后用含有MN58b的培养基替换培养基。72小时后,细胞如前所述进行处理。 细胞凋亡检测[1] 在浓度逐渐增加的MN58b存在下,将细胞(每孔5×104个)接种在6孔板中。24和48小时后,用PBS洗涤细胞,重新悬浮在膜联蛋白V结合缓冲液中,并在黑暗中与APC-Annexin V一起孵育15至20分钟。加入DAPI 15分钟,在FACS Canto II流式细胞仪中评估存活率。使用FlowJo软件对结果进行量化。 药物协同试验[1] 将细胞(每孔2×104个)接种在24孔板中。24小时后,取出培养基,加入药物(MN58b、吉西他滨、奥沙利铂和5-FU)72小时,单独或联合使用,剂量范围根据之前估计的每种药物的IC50。确定存活率,并使用Chou和Talalay方法以及Calcusyn软件计算组合指数(CI)值。CI为0.9-1.1表示加性效应,CI<0.9表示协同作用,CI>1.1表示拮抗作用。 细胞培养和治疗。[2] MDA-MB-231和HT29癌症细胞系在添加10%FCS、80单位/mL青霉素和80μg/mL链霉素的DMEM中培养,温度37°C,5%CO2。使用96孔板在200μL中以1×103的接种密度测量MDA-MB-231细胞的细胞生长抑制(96小时),通过磺基罗丹明B测定来评估IC50。MDA-MB-231细胞在37°C下用相当于5×IC50(6μmol/L)的药理学活性浓度的MN58b处理4、13、19、30和48小时,或用6μmol/L ACG20b(一种非活性类似物)处理48小时,HT29细胞在37℃下用2.5μmol/L MN58b或2.5μmol/L ACG40b处理48小时。然后对细胞进行胰蛋白酶消化和台盼蓝排斥试验。通过计算处理过的烧瓶中附着的细胞数量并将其与对照烧瓶中附着细胞的数量进行比较,监测处理对细胞数量的影响。[2] 细胞周期分析。[2] 在70%乙醇中固定的细胞(1×106)上对附着和分离的对照细胞和处理过的细胞进行细胞周期分析,在37°C下用柠檬酸盐缓冲盐水中的100μg/mL RNase A处理30分钟,并使用Elite Enhanced System Performance细胞分选仪在488 nm下用4μg/mL碘化丙啶染色。使用WinMdi和Cylchred软件分析细胞仪数据[2]。 |
| 动物实验 |
Animal/Disease Models: MF-1 nude mice with HT29 or MDA-MB-231 cells [2]
Doses: 4 mg/kg Route of Administration: intraperitoneal (ip) injection; base inhibitor activity [2]. one time/day; for 5 days. Experimental Results: Phosphate monoesters diminished Dramatically. HT29 and MDA-MB-231 tumor xenograft models. [2] MF-1 nude mice were injected s.c. in the flank with 0.2 mL of a suspension of HT29 human colon carcinoma cells (2.5 × 107/mL) or MDA-MB-231 human breast carcinoma cells (5 × 107/mL) that had been grown as a monolayer in cell culture. Tumor size was calculated by measuring the length, width, and depth of each tumor using calipers and by using the following formula: l × w × d × (π/6). Once an appropriate tumor size (approximate volume of 500 mm3) was established, mice were randomly divided into two groups: one group was treated with MN58b in saline at 4 mg/kg i.p. once a day for 5 days, and one group was treated with saline alone following the same regimen. In vivo 31P-MRS of HT29 and MDA-MB-231 tumor xenografts. [2] Animals were anesthetized with a single i.p. injection of a Hypnovel/Hypnorm/water (1:1:2) mixture as previously described. Animals were placed in the bore of a Varian 4.7-T nuclear magnetic resonance (NMR) spectrometer and tumors were positioned in the center of a 12-mm two-turn 1H/31P surface coil. Image-selected in vivo spectroscopy–localized 31P-MR spectra of the tumors were obtained at 37°C, as previously described. Briefly, a gradient strength of up to 7.5 × 10−4 T/cm was applied with adiabatic pulses of 800 milliseconds, a 90-degree sincos excitation pulse, and a sech 180 inversion pulse, with a total repetition time of 3 seconds and 600 averages. 31P-MRS of the tumors was carried out before treatment (i.e., day 1) and 4 days after treatment (i.e., day 5). 31P-MR spectra were quantified using the VARiable PROjection program to determine precise chemical shifts and peak integrals as previously described. After the final 31P-MRS study, tumors were freeze clamped and stored at −80°C until analysis. The surface coils used to obtain the 31P-MRS signal from s.c. tumors in vivo were of nonuniform spatial sensitivity; thus, it is not possible to use an internal standard. Thus, the signal intensities observed in the in vivo 31P-MR spectra are expressed as ratios of metabolites. In vivo 1H-MRS of HT29 tumor xenografts. [2] Anesthetized mice were placed in the MR system as described above. Voxels were selected from scout gradient echo images, and localized shimming yielded line widths of the order of 20 to 30 Hz. The PRESS localization method with water suppression was used to detect choline with a repetition time of 2 seconds, 64 transients, and echo times of 20, 68, 136, 272, and 408 milliseconds. For unsuppressed water, 16 transients were acquired with the same acquisition variables as above except with a lower receiver gain. After the final 1H-MRS study, tumors were freeze clamped and stored at −80°C until analysis. MRUI software was used for all spectral processing programs, including preprocessing, fitting, and quantification of peak areas of the observed metabolites. Eddy current correction was done by using the water FID to phase correct the metabolite signal point by point. This corrects the phase of the spectrum without any subjective bias. The last 100 data points were used to calculate root-mean-square noise and correct for direct current offsets. Using the choline and water peak areas as a function of echo time, choline and water T2 curves were fitted to a single exponential decay function and choline, and water intercepts (M0) and T2s were derived from these fits. The Levenberg-Marquardt algorithm was used for optimization. The choline concentration was calculated using tumor water as a reference assuming tumor tissue water was 80% (44 mol/L; ref. 44) and according to the equation. In vivo subcutaneous tumorigenic assay [1] Suit2 028 CHKα-silenced (Sh-3 and Sh-5) and their nontarget counterparts (shNt) cells were grown in 6- to 8-week-old female BALB/c Nu/Nu mice. Cells (2 × 106, 100 μL in PBS) were injected subcutaneously, growth was monitored using an electronic caliper, and volumes calculated using the formula (L × W2 × 0.5). Mice were housed in IVC cages. |
| 参考文献 |
|
| 其他信息 |
Choline kinase α (CHKα) plays a crucial role in the regulation of membrane phospholipid synthesis and has oncogenic properties in vitro. We have analyzed the expression of CHKα in cell lines derived from pancreatic ductal adenocarcinoma (PDAC) and have found increased CHKα expression, associated with differentiation. CHKα protein expression was directly correlated with sensitivity to MN58b, a CHKα inhibitor that reduced cell growth through the induction of apoptosis. Accordingly, CHKα knockdown led to reduced drug sensitivity. In addition, we found that gemcitabine-resistant PDAC cells displayed enhanced sensitivity to CHKα inhibition and, in vitro, MN58b had additive or synergistic effects with gemcitabine, 5-fluorouracil, and oxaliplatin, three active drugs in the treatment of PDAC. Using tissue microarrays, CHKα was found to be overexpressed in 90% of pancreatic tumors. While cytoplasmic CHKα did not relate to survival, nuclear CHKα distribution was observed in 43% of samples and was associated with longer survival, especially among patients with well/moderately differentiated tumors. To identify the mechanisms involved in resistance to CHKα inhibitors, we cultured IMIM-PC-2 cells with increasingly higher concentrations of MN58b and isolated a subline with a 30-fold higher IC50. RNA-Seq analysis identified upregulation of ABCB1 and ABCB4 multidrug resistance transporters, and functional studies confirmed that their upregulation is the main mechanism involved in resistance. Overall, our findings support the notion that CHKα inhibition merits further attention as a therapeutic option in patients with PDAC and that expression levels may predict response. [1]
MN58b is a novel anticancer drug that inhibits choline kinase, resulting in inhibition of phosphocholine synthesis. The aim of this work was to develop a noninvasive and robust pharmacodynamic biomarker for target inhibition and, potentially, tumor response following MN58b treatment. Human HT29 (colon) and MDA-MB-231 (breast) carcinoma cells were examined by proton (1H) and phosphorus (31P) magnetic resonance spectroscopy (MRS) before and after treatment with MN58b both in culture and in xenografts. An in vitro time course study of MN58b treatment was also carried out in MDA-MB-231 cells. In addition, enzymatic assays of choline kinase activity in cells were done. A decrease in phosphocholine and total choline levels (P < 0.05) was observed in vitro in both cell lines after MN58b treatment, whereas the inactive analogue ACG20b had no effect. In MDA-MB-231 cells, phosphocholine fell significantly as early as 4 hours following MN58b treatment, whereas a drop in cell number was observed at 48 hours. Significant correlation was also found between phosphocholine levels (measured by MRS) and choline kinase activities (r2 = 0.95, P = 0.0008) following MN58b treatment. Phosphomonoesters also decreased significantly (P < 0.05) in both HT29 and MDA-MB-231 xenografts with no significant changes in controls. 31P-MRS and 1H-MRS of tumor extracts showed a significant decrease in phosphocholine (P < or = 0.05). Inhibition of choline kinase by MN58b resulted in altered phospholipid metabolism both in cultured tumor cells and in vivo. Phosphocholine levels were found to correlate with choline kinase activities. The decrease in phosphocholine, total choline, and phosphomonoesters may have potential as noninvasive pharmacodynamic biomarkers for determining tumor response following treatment with choline kinase inhibitors. [2] |
| 分子式 |
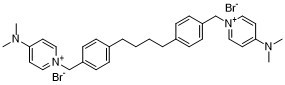
C32H40BR2N4
|
|---|---|
| 分子量 |
640.4948
|
| 精确质量 |
640.159
|
| 元素分析 |
C, 60.01; H, 6.30; Br, 24.95; N, 8.75
|
| CAS号 |
203192-01-2
|
| 相关CAS号 |
203192-01-2 (bromide);730930-74-2 (cation);
|
| PubChem CID |
52952144
|
| 外观&性状 |
White to off-white solid powder
|
| tPSA |
14.2
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
11
|
| 重原子数目 |
38
|
| 分子复杂度/Complexity |
523
|
| 定义原子立体中心数目 |
0
|
| SMILES |
[Br-].[Br-].[N+]1(C([H])=C([H])C(=C([H])C=1[H])N(C([H])([H])[H])C([H])([H])[H])C([H])([H])C1C([H])=C([H])C(=C([H])C=1[H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C1C([H])=C([H])C(=C([H])C=1[H])C([H])([H])[N+]1C([H])=C([H])C(=C([H])C=1[H])N(C([H])([H])[H])C([H])([H])[H]
|
| InChi Key |
YLHINOUDZGYMNO-UHFFFAOYSA-L
|
| InChi Code |
InChI=1S/C32H40N4.2BrH/c1-33(2)31-17-21-35(22-18-31)25-29-13-9-27(10-14-29)7-5-6-8-28-11-15-30(16-12-28)26-36-23-19-32(20-24-36)34(3)4;;/h9-24H,5-8,25-26H2,1-4H3;2*1H/q+2;;/p-2
|
| 化学名 |
1-[[4-[4-[4-[[4-(dimethylamino)pyridin-1-ium-1-yl]methyl]phenyl]butyl]phenyl]methyl]-N,N-dimethylpyridin-1-ium-4-amine;dibromide
|
| 别名 |
MN58b bromide; MN 58b; MN58b; 203192-01-2; 1-[[4-[4-[4-[[4-(dimethylamino)pyridin-1-ium-1-yl]methyl]phenyl]butyl]phenyl]methyl]-N,N-dimethylpyridin-1-ium-4-amine;dibromide; 1,1'-((Butane-1,4-diylbis(4,1-phenylene))bis(methylene))bis(4-(dimethylamino)pyridin-1-ium) bromide; Pyridinium, 1,1'-[1,4-butanediylbis(4,1-phenylenemethylene)]bis[4-(dimethylamino)-, bromide (1:2); 4-(dimethylamino)-1-({4-[4-(4-{[4-(dimethylamino)pyridin-1-ium-1-yl]methyl}phenyl)butyl]phenyl}methyl)pyridin-1-ium dibromide; CHEMBL1771545; MN58b?; MN 58b; MN-58b
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~14.7 mg/mL (~23 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1.47 mg/mL (2.30 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 14.7 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1.47 mg/mL (2.30 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 14.7mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5613 mL | 7.8065 mL | 15.6130 mL | |
| 5 mM | 0.3123 mL | 1.5613 mL | 3.1226 mL | |
| 10 mM | 0.1561 mL | 0.7807 mL | 1.5613 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。