| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
Momelotinib (CYT387) 的 IC50 为 1400 nM,可抑制由 IL-3 触发的亲代 Ba/F3 细胞 (Ba/F3-wt) 的增殖。此外,Momelotinib (CYT387) 的 IC50 为 200,可减少由 JAK2 或 MPL 信号传导激活的细胞系的增殖,例如 Ba/F3-MPLW515L 细胞、CHRF-288-11 细胞和 Ba/F3-TEL-JAK2细胞。 700 nM、1 nM 和 nM。此外,已证明,莫莫替尼 (CYT387) 的 IC50 为 2 μM–4 μM,在体外也能有效抑制 JAK2V617F 阳性 PV 个体的红细胞集落形成 [1]。 momelotinib (CYT387) 可抑制 IGF-1 和 IL-6 诱导的 Ras/MAPK 和 PI3K/AKT 激活。此外,在原发性多发性骨髓瘤 (MM) 细胞中,莫莫替尼 (CYT387) 作为单一药物可促进细胞凋亡,并与传统抗 MM 药物 PS-341 和 L-PAM 产生协同作用 [2]。
|
||
|---|---|---|---|
| 体内研究 (In Vivo) |
Momelotinib (CYT387) 可以纠正 MPN 小鼠模型中的血细胞比容、脾脏大小、白细胞计数和炎症细胞因子的生理水平 [3]。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Momelotinib is rapidly absorbed following oral administration with a bioavailability of 97%. The mean (%CV) steady-state Cmax is 479 ng/mL (61%), and the mean (%CV) AUC is 3,288 ng x h/mL (60%) at the maximum recommended dosage. Momelotinib exposure (i.e., Cmax and AUC) increases dose proportionally from 100 mg to 300 mg (0.5 to 1.5 times the maximum recommended dosage), but less than dose-proportional at doses from 400 mg to 800 mg (two to four times the maximum recommended dosage). There is no clinically significant accumulation. The Tmax at steady state is two hours (Q1: 1 hour; Q3: 3 hours) post-dose. No clinically significant differences in momelotinib pharmacokinetics were observed following administration of either a high-fat meal (800 kcal; 50% fat) or low-fat meal (400 kcal; 20% fat) in healthy subjects. Momelotinib is primarily eliminated in feces and, to a lesser extent, in urine. Following a single oral dose of radiolabeled momelotinib in healthy subjects, about 69% of the total radioactive dose was recovered in fecesm with M14 accounting for 21.4% of the dose, momelotinib and M21 each accounting for 13%, and other 12 metabolites accounting for the remaining 22%. About 28% of radioactivity was recovered in urine, with M21 being the major species. The mean (%CV) apparent volume of distribution at steady-state is 984 L (118%). The mean (%CV) clearance is clearance is 103 L/h (87%). Metabolism / Metabolites Momelotinib is metabolized by multiple cytochrome P450 (CYP) enzymes, including CYP3A4 (36%), CYP2C8 (19%), CYP2C9 (17%), CYP2C19 (19%), and CYP1A2 (9%). M21 is initially formed via oxidation of the morpholine ring by the same CYP enzymes, followed by metabolism via aldehyde oxidase. M21 is a major metabolite in humans that retains approximately 40% of the pharmacological activity of the parent. The mean ratio of M21 to momelotinib for AUC ranged from 1.4 to 2.1. Momelotinib can undergo amide hydrolysis, N-dealkylation, nitrile hydrolysis, nitrile oxidation, and glucuronidation. Biological Half-Life The elimination half-life of momelotinib and the M21 metabolite is four to eight hours. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In the published preregistration clinical trials of momelotinib, rates of serum ALT or AST elevations ranged from 21% to 31% and were above 5 times the upper limit of normal (ULN) in 0.5% to 2.0%, and above 20 times ULN in 0.5%. Two of 448 momelotinib treated patients evaluated in the safety cohort developed clinically apparent, but self-limiting liver injury with jaundice. A third patient developed liver injury with jaundice that appeared to be due to reactivation of hepatitis B. The liver injury was typically hepatocellular without immune allergic or autoimmune features, arising after 2 to 4 months of therapy, and resolving soon after drug discontinuation. Peak ALT elevations ranged from 308 to 1178 U/L and peak bilirubin from 2.3 to 7.0 mg/dL. There were no deaths from hepatic failure. Since its approval and more widespread clinical use, there have been no further reports of serum enzyme or bilirubin elevations or instances of clinically apparent liver injury, but it has been available for a limited time only. Likelihood score: D (possible cause of clinically apparent liver injury including reactivation of hepatitis B). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of momelotinib during breastfeeding. Because momelotinib is 91% bound to plasma proteins, the amount in milk is likely to be low. The manufacturer recommends that breastfeeding be discontinued during momelotinib therapy and for at least 1 week after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Momelotinib is 91% bound to plasma proteins in healthy volunteers. |
||
| 参考文献 |
|
||
| 其他信息 |
Pharmacodynamics
Momelotinib inhibits Janus Kinase 1 and 2 (JAK1/JAK2) with an IC50 of 11 and 18 nM, respectively. It also inhibits JAK3 (IC50 = 155 nM) and tyrosine kinase 2 (TYK2) (IC50 = 17 nM) with less selectivity. Momelotinib inhibited STAT3 phosphorylation in whole blood from patients with myelofibrosis (MF). Maximal inhibition of STAT3 phosphorylation occurred two hours after momelotinib dosing, which persisted for at least six hours. Iron availability and erythropoiesis were assessed by analysis of circulating hepcidin concentrations: an acute and sustained reduction of circulating hepcidin was observed for the duration of the 24-week administration of momelotinib to patients with MF. |
| 分子式 |
C23H22N6O2
|
|---|---|
| 分子量 |
414.46
|
| 精确质量 |
414.18
|
| CAS号 |
1056634-68-4
|
| 相关CAS号 |
Momelotinib sulfate;1056636-06-6;Momelotinib Mesylate;1056636-07-7
|
| PubChem CID |
25062766
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 折射率 |
1.646
|
| LogP |
1.22
|
| tPSA |
103.17
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
31
|
| 分子复杂度/Complexity |
615
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
ZVHNDZWQTBEVRY-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C23H22N6O2/c24-10-12-25-22(30)18-3-1-17(2-4-18)21-9-11-26-23(28-21)27-19-5-7-20(8-6-19)29-13-15-31-16-14-29/h1-9,11H,12-16H2,(H,25,30)(H,26,27,28)
|
| 化学名 |
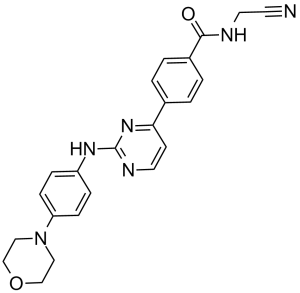
N-(cyanomethyl)-4-(2-((4-morpholinophenyl)amino)pyrimidin-4-yl)benzamide.
|
| 别名 |
LM-1149 , CYT-11387; LM 1149 , CYT 11387; LM1149 , CYT11387; CYT-387; Momelotinib; Momelotinib free base; CYT387; CYT 387; Ojjaara
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.03 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 30% PEG400+0.5% Tween80+5% propylene glycol: 30 mg/mL 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4128 mL | 12.0639 mL | 24.1278 mL | |
| 5 mM | 0.4826 mL | 2.4128 mL | 4.8256 mL | |
| 10 mM | 0.2413 mL | 1.2064 mL | 2.4128 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02244489 | Terminated | Drug: Momelotinib (MMB) Drug: Capecitabine |
Relapsed/Refractory Metastatic Pancreatic Ductal Adenocarcinoma |
Sierra Oncology LLC - a GSK company |
November 5, 2014 | Phase 1 |
| NCT02206763 | Terminated | Drug: Momelotinib (MMB) Drug: Erlotinib |
EGFR Mutated EGFR TKI Naive Metastatic NSCLC |
Sierra Oncology LLC - a GSK company |
October 16, 2014 | Phase 1 |
| NCT01998828 | Terminated | Drug: Larotrectinib Sulfate Procedure: Bone Scan |
Drug: Momelotinib | Polycythemia Vera Essential Thrombocythemia |
February 19, 2014 | Phase 2 |
| NCT02258607 | Terminated | Drug: Momelotinib (MMB) Drug: Trametinib |
Relapsed Metastatic KRAS-Mutated Non-Small Cell Lung Cancer |
Sierra Oncology LLC - a GSK company |
March 11, 2015 | Phase 1 |