| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
VEGFR1 (IC50 = 34 nM); VEGFR2 (IC50 = 13 nM); VEGFR3 (IC50 = 13 nM); FGFR1 (IC50 = 69 nM); FGFR2 (IC50 = 37 nM); FGFR3 (IC50 = 108 nM); PDGFRα (IC50 = 59 nM); PDGFRβ (IC50 = 65 nM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:BIBF1120 抑制 PDGFR α 和 PDGFR β 型的 PDGFR 激酶活性,IC50 值分别为 59 nM 和 65 nM。此外,BIBF1120 抑制 FGFR 亚型,FGFR1、FGFR2 和 FGFR3 的 IC50 分别为 60 nM、37 nM 和 108 nM。 BIBF1120 与激酶结构域氨基端叶和羧基端叶之间缝隙中的 ATP 结合位点结合。吲哚酮支架与铰链区的 Cys919 的主链氮和 Glu917 的主链羰基氧形成两个氢键。 BIBF 1120 抑制 PDGF-BB 刺激的 BRP 增殖,在细胞测定中 EC50 为 79 nM。用 5% 血清加 PDGF-BB 刺激后,浓度低至 100 nM 的 BIBF1120 可阻断 MAPK 的激活。在人血管平滑肌细胞 (HUASMC) 培养物中,BIBF1120 可防止 PDGF-BB 刺激的增殖,EC50 为 69 nM。激酶测定:将 VEGFR2 的细胞质酪氨酸激酶结构域(根据数据库 SWISS-PROT P35968 中保存的序列,残基 797-1355)克隆到与 GST 融合的 pFastBac 中并提取。在存在或不存在 BIBF1120 在 25% DMSO 中进行系列稀释的情况下测定酶活性。每个微量滴定板都包含内部对照,例如空白、最大反应和历史参考化合物。所有孵育均在室温下在旋转摇床上进行。将 10 μL 每种 BIBF1120 稀释液添加到 10 μL 稀释激酶(0.8 μg/mL VEGFR2、10 mM Tris pH 7.5、2 mM EDTA 和 2 mg/mL BSA)中,并预孵育 1 小时。通过添加 30 μL 底物混合物开始反应,其中包含 62.4 mM Tris pH 7.5、2.7 mM DTT、5.3 mM MnCl2、13.3 mM 乙酸镁、0.42 mM ATP、0.83 mg/mL Poly-Glu-Tyr(4:1 )和 1.7 μg/mL Poly-Glu-Tyr(4:1)-生物素并孵育 1 小时。添加 50 μL 250 mM EDTA、20 mM HEPES、pH 7.4 终止反应。将 90 μL 反应混合物转移至链霉亲和素板并孵育 1-2 小时。用 PBS 洗涤 3 次后,添加 EU 标记抗体 PY20(建议在 DELFIA 测定缓冲液中按 1:2000 稀释 0.5 mg/mL 标记抗体)。 DELFIA 洗涤缓冲液洗涤 3 次即可去除过量的检测抗体。然后,在多标签阅读器上测量前 10 分钟,将每个孔与 100 μL DELFIA 增强溶液一起孵育。 [1] 细胞测定:细胞系(HUVEC、HUASMC 和 BRP)用于测定。在添加配体前两小时将 BIBF1120 添加到培养物中。产生细胞裂解物。蛋白质印迹使用标准 SDS-PAGE 方法进行,每泳道上样 50 至 75 μg 蛋白质。增强的化学发光有助于检测。使用单克隆抗体 M3807 和 M8159 分析总丝裂原激活蛋白激酶 (MAPK) 和磷酸化丝裂原激活蛋白激酶 (MAPK)。使用相应的多克隆抗体检测总 Akt,并使用其单克隆抗体分析磷酸化 Akt (Ser473)。单克隆抗体也用于检测裂解的 caspase-3,而 KDR (VEGFR2) 蛋白则使用相应的抗体进行检测。 [1]
激酶选择性特征。[1] 广泛的生化测试揭示了一种独特的、窄范围的激酶,这些激酶在药理学相关浓度下被Nintedanib/BIBF 1120抑制。靶向激酶包括所有三种VEGFR亚型(IC50,13-34 nmol/L)、PDGFRα和PDGFRβ(IC50,59和65 nmool/L)以及FGFR 1、2和3型(IC50分别为69、37和108 nmol/L;表1)。相应的人类和啮齿动物激酶也有类似的抑制作用。此外,BIBF 1120抑制FLT3(抑制急性髓系白血病细胞增殖已在先前显示;参考文献29)以及Src家族成员(Src、Lyn和Lck)。相比之下,受体酪氨酸激酶,如EGFR和HER2、InsR、IGF-IR或细胞周期激酶CDK1、CDK2和CDK4(表1)在低于1000nmol/L的浓度下没有受到抑制。 内皮细胞的信号通路、增殖和存活。用NintedanibBIBF 1120处理来源于脐静脉(HUVEC)和皮肤微血管(HSMEC)的VEGF刺激的内皮细胞,可抑制细胞增殖和凋亡(EC50,<10 nmol/L;表2),并在此之前抑制MAPK和Akt磷酸化(图2A)。抑制bFGF刺激的HUVEC增殖需要更高的药物浓度(EC50,290 nmol/L),尽管在低至100 nmol/L的浓度下,MAPK和Akt的激活至少部分受到抑制。在VEGF刺激和bFGF刺激的HUVEC中,凋亡标志物切割的caspase-3以浓度依赖的方式上调,在50 nmol/L BIBF 1120存在的情况下,TUNEL染色测量的凋亡HUVEC细胞的比例从对照细胞的2%增加到28%(补充图S1A)。 对周细胞和平滑肌细胞的影响。[1] 众所周知,周细胞对血管成熟和稳定很重要,可以表达PDGFR(30)Nintedanib/BIBF 1120抑制PDGF-BB刺激的BRPs增殖,EC50为79 nmol/L(表2),这与生化激酶抑制数据基本一致。信号通路分析表明,浓度低至100nmol/L的BIBF 1120可以阻断5%血清加PDGF-BB刺激后MAPK的激活。用5%血清加bFGF刺激BRP可以阻断MAPK磷酸化,但不是浓度依赖性的(图2B)。在用PDGF-BB或bFGF刺激至100nmol/L的浓度后,BIBF 1120明显抑制了Akt的激活;有趣的是,这种途径的抑制并没有导致切割的半胱氨酸天冬氨酸蛋白酶-3的增加。 在人血管平滑肌细胞(HUASMC)的培养物中,Nintedanib/BIBF 1120抑制了PDGF-BB刺激的增殖,EC50为69 nmol/L(表2),MAPK激活在低至100 nmol/L的浓度下受到抑制。用bFGF刺激的HUASMC的细胞裂解物在300 nmol/L以上的浓度下显示出对MAPK激活的抑制。Akt的磷酸化在bFGF中完全被阻断,而PDGF-BB在BIBF 1120低至100 mmol/L的浓度下被阻断。此外,在用BIBF 1120处理的bFGF刺激的HUASMC中,凋亡标志物切割的caspase-3上调(图2C)。 持续阻断VEGFR。[1] 为了确定Nintedanib/BIBF 1120对VEGFR-2的抑制持续时间,对VEGFR-2-转染的NIH3T3细胞进行了脉冲追逐实验(31)。将细胞暴露于50 nmol/L BIBF 1120 1小时,用PBS彻底清洗,在培养基中孵育8、24或32小时,然后用VEGF刺激10分钟。免疫沉淀后细胞裂解物的蛋白质印迹分析显示,在去除BIBF 1120后,受体磷酸化的抑制持续了至少32小时(补充图S1B)。 三氟尿苷和Nintedanib对体外培养的结直肠癌癌症细胞系的联合作用[2] 基于DLD-1、HT-29和HCT116细胞(图1A-C)单独使用三氟尿苷或尼达尼布的72小时生长抑制曲线,使用三条等效曲线(模式I、模式IIa和模式IIb)绘制等深线图。根据可用的剂量反应曲线,我们分析了两种药物在IC50点的联合作用。三氟尿苷在DLD-1、HT-29和HCT116细胞中的IC50值分别为4.3×10−6、3.8×10−6和1.8×10−2 M,而尼达尼布的相应IC50值分别是3.4×10−3、1.4×10−4和2.5×10−5 M。在DLD-1和HT-29细胞中,暴露于联合处理72小时会产生相加效应(图1A和B)。在HCT116细胞中,上述组合处理产生了亚加性效应(图1C)。 |
| 体内研究 (In Vivo) |
在迄今为止测试的所有肿瘤模型中,包括在裸鼠中生长的人类肿瘤异种移植物和同基因大鼠肿瘤模型,BIBF1120在耐受良好的剂量(每天口服25-100 mg/kg)下具有高度活性。这在 3 天后肿瘤灌注的磁共振成像、5 天后血管密度和血管完整性降低以及深度生长抑制中显而易见。 [2]
BIBF 1120/尼达尼布影响肿瘤血管密度和周细胞。[1] 为了确认BIBF 1120影响肿瘤血管系统,用载体对照或BIBF 1120以100mg/kg的剂量连续五天治疗已建立FaDu异种移植物的小鼠。最后一次施用后,使用Meca 32和PDGFRβ特异性抗体对内皮细胞和周细胞进行免疫组化解剖和分析(图3B)。与对照肿瘤相比,用BIBF 1120治疗的小鼠异种移植物中的血管密度降低了76%(图3C;P<0.001)。用BIBF 1120治疗5天后,PDGFRβ阳性壁细胞的定量显示减少了64%(图3C;P<0.001)。对照组和BIBF 1120治疗小鼠的肿瘤切片中Meca 32和PDGFRβ的双重免疫荧光染色显示,对照组小鼠的Meca 32阳性内皮细胞和PDGFRα阳性周细胞明显相关(图3D,上图),而在BIBF 1120处理的小鼠中,与分隔肿瘤结节的瘤周肿瘤基质相比,Meca 32阴性和PDGFRγ阳性细胞主要在瘤内室中明显减少(图3D:右下两条虚线之间的区域)。在高倍镜下,在对照小鼠的肿瘤样本中可以看到Meca 32阳性和PDGFRβ阳性细胞之间的紧密关联,但在BIBF 1120治疗的肿瘤样本(图3D,左上角和左下角的箭头)中则没有。这些数据不仅显示BIBF 1120治疗后Meca 32阳性和PDGFRβ阳性细胞减少,而且在治疗5天后发现的大多数肿瘤血管中,这两种细胞类型之间的紧密联系也消失了。 体内抗肿瘤活性与小鼠独特的药代动力学特征和良好的耐受性有关。[1] 用50或100mg/kg的已建立的FaDu肿瘤异种移植物对小鼠进行连续的每日一次口服治疗,可显著抑制肿瘤生长,治疗组与对照组的T/C值分别为27%和11%(图4A)。BIBF 1120/尼达尼布即使在高剂量组中也具有良好的耐受性,在治疗期间没有明显的体重减轻。如补充表S1所述,在人肾细胞癌(图4B;Caki-1)、结直肠(HT-29)、卵巢(SKOV-3)、非小细胞肺(Calu-6)和前列腺癌(PAC-120)的异种移植物模型中也观察到明显的肿瘤生长抑制。此外,在同基因大鼠胶质母细胞瘤模型(细胞系GS-9L)中,观察到50、25和10mg/kg的疗效,T/C值分别为30%、45%和74%(补充表S1)。小鼠口服给药后的药代动力学研究(图4C)显示,给药后1小时的最大血浆浓度约为1000 nmol/L,给药24小时的谷血浆水平低于8 nmol/L。这种独特的药代动力学特征可以通过甲酯裂解BIBF 1120的快速代谢来解释,导致产生含有游离酸残基的主要代谢产物BIBF 1202(数据未显示)。 TFTD/Nintedanib联合治疗体内抗肿瘤疗效[2] 评估了TFTD单药治疗、Nintedanib单药治疗以及TFTD和Nintedanib联合治疗在人类结直肠癌癌症异种移植物模型中的体内疗效。 携带DLD-1肿瘤的裸鼠连续14天接受150mg/kg TFTD、40mg/kg<strong>尼达尼布</strong>或TFTD和尼达尼布联合治疗。在第15天,TFTD单药治疗和尼达尼布单药治疗导致体内肿瘤生长显著减少(P<0.01)(图2A)。此外,联合疗法比两种单一疗法表现出更大的抗肿瘤活性。 在荷肿瘤的裸鼠中评估上述治疗的疗效,所述肿瘤来源于耐5-FU的人结直肠癌癌症细胞DLD-1/5-FU(图2C)。TFTD单药治疗和尼达尼布单药治疗导致体内肿瘤生长显著减少(P<0.01)。两种单一疗法的抗肿瘤效果在5-FU耐药DLD-1细胞和亲本DLD-1细胞之间相似。这表明DLD-1/5-FU和任何一种单一疗法之间都没有发生交叉耐药性。与两种单一疗法相比,TFTD/尼达尼布联合疗法对DLD-1/5-FU表现出更大的抗肿瘤活性。因此,联合治疗对DLD-1/5-FU(肿瘤生长抑制率72.8%)和DLD-1(肿瘤生长抑制剂率61.5%)肿瘤显示出类似的抗肿瘤作用(数据未显示)。 |
| 酶活实验 |
体外激酶活性测定。[1]
将VEGFR-2的细胞质酪氨酸激酶结构域(根据数据库SWISS-PROT P35968中的序列,残基797-1355)克隆到与GST融合的pFastBac中,并按照补充方法进行提取。在标准条件下,使用无规聚合物(Glu/Tyr 4:1)并在100μmol/L ATP的存在下测定酶活性(详见补充方法)。对于所有其他激酶测定,将受体的整个细胞质结构域(从跨膜末端到COOH末端)克隆到含有GST的pFastBac载体中,并在标准条件下进行测定。 体外VEGFR-2激酶测定[3] 将VEGFR-2的细胞质激酶结构域(根据数据库SWISS-PROT P35968中的序列,残基797至1335)克隆到与谷胱甘肽-S-转移酶(GST)融合的pFastBac中。GST融合蛋白在SF-9昆虫细胞中表达,并用HEPEX(20 mM HEPES pH 7.4,100 mM NaCl,10 mM ss甘油磷酸,10 mM对硝基苯基磷酸,30 mM NaF,5 mM EDTA,5%甘油,1%Triton X-100,1 mM Na3VO4,0.1%SDS,0.5μg/mL pepstatin A,2.5μg/mL 3,4-二氯异香豆素,2.5μg/mL反式环氧琥珀酰-l-亮酰基-l-酰胺丁烷,抑肽酶20 KIU/mL,亮肽2μg/mL,苯甲脒1 mM和0.002%PMSF)提取。在25%DMSO中进行的抑制剂系列稀释存在或不存在的情况下测定酶活性。每个微量滴定板都包含内部对照,如空白、最大反应和历史参考化合物。所有培养均在室温下在旋转振荡器上进行。将10μL每种抑制剂稀释液加入10μL稀释的激酶(0.8μg/mL VEGFR-2、10 mM Tris pH 7.5、2 mM EDTA、2 mg/mL BSA)中,预孵育1小时。通过加入30μL底物混合物开始反应,该底物混合物含有62.4 mM Tris pH7.5、2.7 mM DTT、5.3 mM MnCl2、13.3 mM乙酸镁、0.42 mM ATP、0.83 mg/mL Poly-Glu-Tyr(4:1)和1.7μg/mL Poly-Glu-Ter(4:1。然后将90μL停止的溶液转移到链霉抗生物素蛋白板上,孵育1-2小时。用PBS洗涤三次EU标记的抗体后,加入PY20(建议稀释1:2000的0.5 mg/mL标记抗体在DELFIA测定缓冲液中)。通过三次洗涤DELFIA洗涤缓冲液去除过量的检测抗体。然后在多标记阅读器VICTOR上测量前10分钟,将每个孔与100μL DELFIA增强溶液一起孵育。IC50值通过使用具有可变斜率的非线性回归分析的S形曲线分析程序计算。 含有 VEGFR2 胞质酪氨酸激酶结构域(残基 797-1355,基于数据库 SWISS-PROT P35968 中存储的序列)的 pFastBac 克隆与 GST 融合并提取。酶活性测定在含有或不含连续稀释的 Nintedanib/BIBF1120 的 25% DMSO 中进行。每个微量滴定板上都有内部对照,包括空白、最大反应和历史参考化合物。在旋转摇床上,所有孵育均在室温下进行。将 10 μL 稀释激酶(0.8 μg/mL VEGFR2、10 mM Tris pH 7.5、2 mM EDTA 和 2 mg/mL BSA)与 10 μL 每种 BIBF1120 稀释液预孵育一小时。添加 30 μL 底物混合物,其中含有 13.3 mM 乙酸镁、6.2.4 mM Tris pH 7.5、2.7 mM DTT、5.3 mM MnCl2、0.42 mM ATP、0.83 mg/mL Poly-Glu- Tyr(4:1) 和 1.7 μg/mL Poly-Glu-Tyr(4:1)-生物素引发反应,然后孵育一小时。将 90 μL 反应混合物置于链霉亲和素板上并孵育 1 至 2 小时。添加 50 μL 250 mM EDTA、20 mM HEPES 和 pH 7.4 终止反应。用 EU 标记抗体用 PBS 洗涤 3 次后,添加 PY20(建议在 DELFIA 测定缓冲液中按 1:2000 稀释 0.5 mg/mL 标记抗体)。使用 3 次 DELFIA 洗涤缓冲液洗涤以除去多余的检测抗体。然后将 DELFIA 增强溶液 (100 μL) 在每个孔中孵育 10 分钟,然后在多标签阅读器上进行测量。 |
| 细胞实验 |
该测定使用细胞系 BRP、HUASMC 和 HUVEC。在添加配体之前两小时向培养物补充 BIBF1120。产生细胞裂解物。标准 SDS-PAGE 技术用于蛋白质印迹,每泳道上样 50–75 μg 蛋白质。改进的化学发光有助于检测。单克隆抗体 M3807 和 M8159 用于分析总丝裂原激活蛋白激酶 (MAPK) 和磷酸化丝裂原激活蛋白激酶 (MAPK)。使用磷酸化 Akt (Ser473) 的单克隆抗体对其进行分析,而相应的多克隆抗体则用于检测总 Akt。虽然相应的抗体用于检测 KDR (VEGFR2) 蛋白,但单克隆抗体也用于检测裂解的 caspase-3。
药物处理细胞中细胞信号级联的抑制。[1] 如上所述培养HUVEC、HUASMC和BRP。在加入配体前两小时,将Nintedanib/BIBF 1120加入培养物中。根据标准方案产生细胞裂解物。使用标准SDS-PAGE方法进行蛋白质印迹,每条泳道装载50至75μg蛋白质,通过增强化学发光检测。使用单克隆抗体分析总丝裂原活化蛋白激酶(MAPK)和磷酸化MAPK。 细胞毒性试验和体外联合作用评价[2] 用结晶紫法测定药物的细胞毒性。将细胞(2000-4000)在96孔微孔板中培养24小时,每孔含100µl培养基。将三氟啶和尼达尼布以10mM的浓度溶解在二甲亚砜中,并在无菌条件下使用培养基制备相应的溶液。将总共100µl的药物溶液(三氟尿苷:0.18–10µM;尼达尼布:0.18-10µM)加入培养基中。将平板温育72小时后,取出培养基,用4%戊二醛固定细胞30分钟。用0.1%结晶紫染色固定细胞2分钟,洗涤并溶解在0.05 M NaH2PO4/50%乙醇中。使用酶标仪在540nm波长下测量吸光度。 使用等深线图法分析了三氟尿苷和尼达尼布组合的细胞毒性作用。基于单独使用三氟尿苷和单独使用尼达尼布的生长抑制曲线,绘制了总共3条等效曲线(模式I、IIa和IIb)。三条曲线所包围的总面积代表了一个“可加性包络”。当实验观察到的IC50值包含在封套的左侧时,药物治疗的组合被认为显示出超加性(协同)相互作用,而当IC50值包含在内时,该组合被认为是加性的。当IC50值包含在封套的右侧并且在虚线方格内时,该组合被认为是亚加性的。最后,当IC50值超出平方时,该组合被认为具有保护作用。 |
| 动物实验 |
For the assay, athymic NMRI-nu/nu female mice weighing between 21 and 33 grams are five to six weeks old. Following their acclimation, mice are injected with 1 to 5×106 (in 100 μL) of SKOV-3, FaDu, Caki-1, H460, HT-29, or PAC-120 cells subcutaneously into their right flank. Following their acclimation, 5×106 (in 100 μL) GS-9L cells are subcutaneously injected into the right flank of F344 Fischer rats. Blood is extracted from the retroorbital plexus of mice at predetermined intervals for pharmacokinetic analysis, and plasma is examined using high performance liquid chromatography-mass spectrometry methodology[1].
In vivo tumor models.[1] Five-week-old to 6-wk-old athymic NMRI-nu/nu female mice (21–31 g) were used. After acclimatization, mice were inoculated with 1 to 5 × 106 (in 100 μL) FaDu, Caki-1, SKOV-3, H460, HT-29, or PAC-120 cells s.c. into the right flank of the animal. F344 Fischer rat were injected with 5 × 106 (in 100 μL) GS-9L cells s.c. into the right flank of the animal. For pharmacokinetic analysis, blood was isolated at indicated time points from the retroorbital plexus of mice and plasma was analyzed using high performance liquid chromatography–mass spectrometry methodology. [1] TFTD was prepared by mixing trifluridine and TPI at a molar ratio of 1:0.5 in 0.5% HPMC solution. The dose of TFTD was expressed on the basis of the trifluridine content. TFTD was administered orally from day 1 to 14, twice a day at 6-h intervals at the reported effective dose (150 mg/kg/day). Nintedanib was administered orally from day 1 to 14, twice a day at 6-h intervals at the reported effective dose (40 mg/kg/day) (14,24). The vehicle solution that consisted of 0.5% HPMC solution was administered at 10 ml/kg to the control mouse group, following the same administration schedules as for the test drugs [2]. |
| 药代性质 (ADME/PK) |
Pharmacokinetic studies after p.o. application to mice (Fig. 4C) revealed a maximal plasma concentration of ∼1,000 nmol/L at 1 hour and trough plasma levels below 8 nmol/L at 24 hours postadministration. This distinctive pharmacokinetic profile can be explained by the rapid metabolization of Nintedanib/BIBF 1120 by methyl ester cleavage, resulting in the generation of the main metabolite BIBF 1202 containing a free acid residue (data not shown). [1]
Rapid in vivo effects on tumor perfusion and permeability detected by DCE-MRI. [1] Human FaDu (squamous cell carcinoma of the head and neck) xenografts growing in nude mice were analyzed by DCE-MRI using gadolinium contrast agent before and 72 hours after initiation of daily p.o. treatment with Nintedanib/BIBF 1120 at 100 mg/kg. Tumor perfusion and vascular permeability was readily visible in the initial MRI scan and clearly reduced after 3 days of treatment (Fig. 3A); quantitation of the KTRANS value showed a significant decrease in Nintedanib/BIBF 1120–treated tumors compared with both baseline values and untreated controls (Fig. 3A). Because of their cellular potency and attractive selectivity profiles, compounds 2 and 3 were selected for in vivo testing. Both compounds yielded good plasma levels 2 h after oral administration to mice and were almost completely cleared from plasma 24 h after administration (Table 3). As shown for the lead structures, none of the compounds inhibited the proliferation of VEGF-independent cell lines at similar concentrations to those tested in HUVEC cells (EC50 > 1 μM), particularly HeLa, Calu-6, and FaDu tumor cell lines. Preclinical Pharmacokinetics Relevant to Human Pharmacokinetics [4] The pharmacokinetics and drug metabolism of nintedanib (dosed via intravenous [IV] infusion or oral gavage) were studied in several animal species. Mean plasma protein binding of nintedanib was > 97% in mice and rats, 91–93% in monkeys, and 98% in humans, over a concentration range of 50–2000 ng/mL [13, 17, 31]. Albumin was the major binding protein. Following administration of [14C]-radiolabelled nintedanib to rats, radioactivity was widely distributed into most of the tissues (except the central nervous system [CNS]). Repeated oral dosing ([14C]-radiolabelled nintedanib 30 mg/kg) for 13 days showed a slight accumulation in some tissues, although a similar accumulation in plasma concentrations was not apparent. Clinical Pharmacokinetics [4] The clinical pharmacokinetics of nintedanib monotherapy were investigated in healthy subjects, volunteers with hepatic impairment, and in patients with IPF or various advanced types of cancer. In healthy volunteers, only single-dose administration was performed. Key pharmacokinetic parameters following single and steady-state twice-daily dosing of nintedanib in patients with advanced cancer are presented in Table 2. The pharmacokinetics of nintedanib were further characterised by two successive population pharmacokinetic (PopPK) analyses, the first based on combined pharmacokinetic data from NSCLC (n = 849) and IPF (n = 342) patients and the second in IPF patients only (n = 933) enrolled in the phase II and III trials. For comparison, Table 3 gives key pharmacokinetic parameters after multiple dosing of nintedanib to typical patients with IPF or NSCLC based on the PopPK analyses. These results show that the key pharmacokinetic parameters are consistent across the two patient populations. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the clinical use of nintedanib during breastfeeding. Because nintedanib is more than 97% bound to plasma proteins, the amount in milk is likely to be low. However, its half-life is about 9.7 hours and it might accumulate in the infant. The manufacturer recommends that breastfeeding be discontinued during nintedanib therapy. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. In Vitro Drug–Drug Interaction Victim and Perpetrator Properties [4] Several in vitro metabolism, transport and drug interaction studies were performed to quantitatively assess the drug–drug interaction potential of nintedanib. In vitro studies with human hepatocytes and/or human liver microsomes showed that nintedanib is a minor substrate for cytochrome P450 (CYP) 3A4 isoenzyme and has a very low potential (along with its two major metabolites [BIBF 1202 and BIBF 1202 glucuronide]) to inhibit or induce CYP isoenzymes, including those that are most relevant or genetically polymorphic for drug metabolism in humans (CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19 and CYP3A4). In human liver microsomes, nintedanib was rapidly hydrolysed by esterases (major mechanism) and demethylated by CYP3A4 (minor mechanism) to form the metabolites BIBF 1202 and BIBF 1053, respectively. CYP-dependent metabolism accounted for about 5% compared to about 25% ester cleavage. Thus, drug–drug interactions with nintedanib as a victim of CYP enzyme-modulating agents (e.g. with co-medication with CYP inhibitors or inducers) are considered very unlikely. Furthermore, drug–drug interactions with nintedanib as a perpetrator of CYP enzymes (e.g. nintedanib acting as a CYP enzyme inhibitor or inducer) are also considered very unlikely. Further in vitro data indicated that nintedanib, at clinically relevant concentrations, did not inhibit glucuronidation by uridine 5′-diphospho-glucuronosyltransferase (UDP-glucuronosyltransferase, UGT) 1A1 (UGT1A1) in human liver microsomes. UGT1A1 is responsible for the glucuronidation of the metabolite BIBF 1202 to BIBF 1202 glucuronide in human liver microsomes. In addition, BIBF 1202 was glucuronidated by several intestinal UGTs (UGT1A7, UGT1A8, UGT1A10). A clinically relevant drug–drug interaction based on inhibition of UGT after oral administration of nintedanib is considered less likely as all half-maximal inhibitory concentration (IC50) values are substantially higher than the therapeutic plasma concentrations. In vitro assays using transfected MDCK cells demonstrated that nintedanib is a substrate of the efflux transporter P-gp and weakly inhibits P-gp (Table 1). Studies using cell lines that express different drug transporters showed that nintedanib was not a substrate of organic anion-transporting polypeptide (OATP) 1B1, OATP1B3, OATP2B1, organic cation transporter (OCT) 2, multidrug resistance-associated protein 2 (MRP-2) or the efflux breast cancer resistance protein (BCRP), but was a weak substrate for OCT1. Nintedanib did not inhibit OATP1B1-, OATP1B3-, OATP2B1-, OCT1-, OCT2-, P-gp- or BRCP-mediated transport at clinically relevant concentrations. |
| 参考文献 |
|
| 其他信息 |
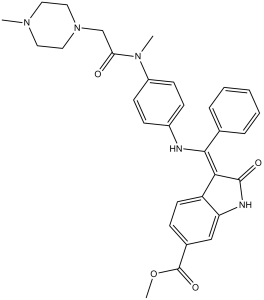
Nintedanib is a member of the class of oxindoles that is a kinase inhibitor used (in the form of its ethylsulfonate salt) for the treatment of idiopathic pulmonary fibrosis and cancer. It has a role as an antineoplastic agent, a tyrosine kinase inhibitor, a vascular endothelial growth factor receptor antagonist, a fibroblast growth factor receptor antagonist and an angiogenesis inhibitor. It is an aromatic ester, a methyl ester, a member of oxindoles, an enamine, an aromatic amine, an aromatic amide and a N-alkylpiperazine. It is a conjugate base of a nintedanib(1+).
Nintedanib is a Kinase Inhibitor. The mechanism of action of nintedanib is as a Protein Kinase Inhibitor. See also: Nintedanib (annotation moved to). Drug Indication Ofev is indicated in adults for the treatment of Idiopathic Pulmonary Fibrosis (IPF). Inhibition of tumor angiogenesis through blockade of the vascular endothelial growth factor (VEGF) signaling pathway is a novel treatment modality in oncology. Preclinical findings suggest that long-term clinical outcomes may improve with blockade of additional proangiogenic receptor tyrosine kinases: platelet-derived growth factor receptors (PDGFR) and fibroblast growth factor receptors (FGFR). BIBF 1120 is an indolinone derivative potently blocking VEGF receptor (VEGFR), PDGFR and FGFR kinase activity in enzymatic assays (IC(50), 20-100 nmol/L). BIBF 1120 inhibits mitogen-activated protein kinase and Akt signaling pathways in three cell types contributing to angiogenesis, endothelial cells, pericytes, and smooth muscle cells, resulting in inhibition of cell proliferation (EC(50), 10-80 nmol/L) and apoptosis. In all tumor models tested thus far, including human tumor xenografts growing in nude mice and a syngeneic rat tumor model, BIBF 1120 is highly active at well-tolerated doses (25-100 mg/kg daily p.o.), as measured by magnetic resonance imaging of tumor perfusion after 3 days, reducing vessel density and vessel integrity after 5 days, and inducing profound growth inhibition. A distinct pharmacodynamic feature of BIBF 1120 in cell culture is sustained pathway inhibition (up to 32 hours after 1-hour treatment), suggesting slow receptor off-kinetics. Although BIBF 1120 is rapidly metabolized in vivo by methylester cleavage, resulting in a short mean residence time, once daily oral dosing is fully efficacious in xenograft models. These distinctive pharmacokinetic and pharmacodynamic properties may help explain clinical observations with BIBF 1120, currently entering phase III clinical development. [1] Trifluridine/tipiracil (TFTD) is a combination drug that is used for the treatment of metastatic colorectal cancer and was formerly known as TAS-102. It is a combination of two active pharmaceutical compounds, trifluridine, an antineoplastic thymidine-based nucleoside analog, and tipiracil, which enhances the bioavailability of trifluridine in vivo. TFTD is used for the treatment of patients with unresectable advanced or recurrent colorectal cancer that is resistant to standard therapies. In the present study, the anticancer effects of trifluridine in combination with nintedanib, an oral triple angiokinase inhibitor, on human colorectal cancer cell lines were investigated. The cytotoxicity against DLD-1, HT-29, and HCT116 cell lines was determined by the crystal violet staining method. The combination of trifluridine and nintedanib exerted an additive effect on the growth inhibition of DLD-1 and HT-29 cells and a sub-additive effect on HCT116 cells, as determined by isobologram analyses. Subsequently, the human colorectal cancer cell lines were implanted subcutaneously into nude mice to allow the evaluation of the in vivo tumor growth inhibitory effects of TFTD and nintedanib combination therapy. TFTD (150 mg/kg/day) and/or nintedanib (40 mg/kg/day) were orally administered to the mice twice daily from day 1 to day 14. The tumor growth inhibition with combination therapy was 61.5, 72.8, 67.6 and 67.5% for the DLD-1, DLD-1/5-FU, HT-29, and HCT116 xenografts, respectively. This was significantly (P<0.05) higher than the effects of monotherapy with either TFTD or nintedanib. These results demonstrated the effectiveness of the combination of TFTD and nintedanib in the treatment of colorectal cancer xenografts. The concentration of trifluridine incorporated into DNA in the HT-29 and HCT116 tumors was determined by liquid chromatography-tandem mass spectrometry. The incorporation levels following treatment with TFTD and nintedanib for 14 consecutive days were higher than those associated with TFTD treatment alone. The preclinical findings indicate that the combination therapy with TFTD and nintedanib is a promising treatment option for colorectal cancer. [2] Inhibition of tumor angiogenesis through blockade of the vascular endothelial growth factor (VEGF) signaling pathway is a new treatment modality in oncology. Preclinical findings suggest that blockade of additional pro-angiogenic kinases, such as fibroblast and platelet-derived growth factor receptors (FGFR and PDGFR), may improve the efficacy of pharmacological cancer treatment. Indolinones substituted in position 6 were identified as selective inhibitors of VEGF-, PDGF-, and FGF-receptor kinases. In particular, 6-methoxycarbonyl-substituted indolinones showed a highly favorable selectivity profile. Optimization identified potent inhibitors of VEGF-related endothelial cell proliferation with additional efficacy on pericyctes and smooth muscle cells. In contrast, no direct inhibition of tumor cell proliferation was observed. Compounds 2 (BIBF 1000) and 3 (BIBF 1120) are orally available and display encouraging efficacy in in vivo tumor models while being well tolerated. The triple angiokinase inhibitor 3 is currently in phase III clinical trials for the treatment of nonsmall cell lung cancer. [3] Nintedanib is an oral, small-molecule tyrosine kinase inhibitor approved for the treatment of idiopathic pulmonary fibrosis and patients with advanced non-small cell cancer of adenocarcinoma tumour histology. Nintedanib competitively binds to the kinase domains of vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF). Studies in healthy volunteers and in patients with advanced cancer have shown that nintedanib has time-independent pharmacokinetic characteristics. Maximum plasma concentrations of nintedanib are reached approximately 2-4 h after oral administration and thereafter decline at least bi-exponentially. Over the investigated dose range of 50-450 mg once daily and 150-300 mg twice daily, nintedanib exposure increases are dose proportional. Nintedanib is metabolised via hydrolytic ester cleavage, resulting in the formation of the free acid moiety that is subsequently glucuronidated and excreted in the faeces. Less than 1% of drug-related radioactivity is eliminated in urine. The terminal elimination half-life of nintedanib is about 10-15 h. Accumulation after repeated twice-daily dosing is negligible. Sex and renal function have no influence on nintedanib pharmacokinetics, while effects of ethnicity, low body weight, older age and smoking are within the inter-patient variability range of nintedanib exposure and no dose adjustments are required. Administration of nintedanib in patients with moderate or severe hepatic impairment is not recommended, and patients with mild hepatic impairment should be monitored closely and the dose adjusted accordingly. Nintedanib has a low potential for drug-drug interactions, especially with drugs metabolised by cytochrome P450 enzymes. Concomitant treatment with potent inhibitors or inducers of the P-glycoprotein transporter can affect the pharmacokinetics of nintedanib. At an investigated dose of 200 mg twice daily, nintedanib does not have proarrhythmic potential.[4] |
| 分子式 |
C31H33N5O4
|
|---|---|
| 分子量 |
539.62
|
| 精确质量 |
539.253
|
| 元素分析 |
C, 69.00; H, 6.16; N, 12.98; O, 11.86
|
| CAS号 |
656247-17-5
|
| 相关CAS号 |
Nintedanib esylate;656247-18-6;Nintedanib-13C,d3;Nintedanib-d3;1624587-84-3;Nintedanib-d8;1624587-87-6
|
| PubChem CID |
135423438
|
| 外观&性状 |
Yellow solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
742.2±60.0 °C at 760 mmHg
|
| 闪点 |
402.7±32.9 °C
|
| 蒸汽压 |
0.0±2.5 mmHg at 25°C
|
| 折射率 |
1.658
|
| LogP |
2.59
|
| tPSA |
94.22
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
40
|
| 分子复杂度/Complexity |
892
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C(C([H])([H])N1C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C1([H])[H])N(C([H])([H])[H])C1C([H])=C([H])C(=C([H])C=1[H])/N=C(\C1C([H])=C([H])C([H])=C([H])C=1[H])/C1=C(N([H])C2C([H])=C(C(=O)OC([H])([H])[H])C([H])=C([H])C1=2)O[H]
|
| InChi Key |
CPMDPSXJELVGJG-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C31H33N5O4/c1-34-15-17-36(18-16-34)20-27(37)35(2)24-12-10-23(11-13-24)32-29(21-7-5-4-6-8-21)28-25-14-9-22(31(39)40-3)19-26(25)33-30(28)38/h4-14,19,33,38H,15-18,20H2,1-3H3
|
| 化学名 |
methyl 2-hydroxy-3-[N-[4-[methyl-[2-(4-methylpiperazin-1-yl)acetyl]amino]phenyl]-C-phenylcarbonimidoyl]-1H-indole-6-carboxylate
|
| 别名 |
BIBF1120; Nintedanib; BIBF-1120; Intedanib; BIBF 1120; trade name: Vargatef
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 10 mg/mL (18.53 mM) in 50% PEG300 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 10 mg/mL (18.53 mM) in 1% CMC 0.5% Tween-80 (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 View More
配方 3 中的溶解度: 30% PEG400+0.5% Tween80+5% propylene glycol: 30mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8532 mL | 9.2658 mL | 18.5316 mL | |
| 5 mM | 0.3706 mL | 1.8532 mL | 3.7063 mL | |
| 10 mM | 0.1853 mL | 0.9266 mL | 1.8532 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Post-marketing Surveillance of Ofev Capsules in Chronic Fibrosing Interstitial Lung Diseases With a Progressive Phenotype in Japan
CTID: NCT04559581
Phase: Status: Active, not recruiting
Date: 2024-11-18
Cancer Res. 2008 Jun 15;68(12):4774-82. |
 |
Cancer Res. 2008 Jun 15;68(12):4774-82. |