| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg | |||
| 500mg | |||
| Other Sizes |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The pharmacokinetics of nomifensine were studied in 6 subjects aged 23-41 yr, after single oral administration of two 50 mg capsules of nomifensine maleate (Nomival) and 100 mg intravenous injection, and after 2 wk of oral daily administration of 150 mg in capsule form. The determination of free and total nomifensine in plasma and urine was performed by HPLC. Nomifensine was rapidly absorbed from the gastrointestinal tract and peak concentration of free nomifensine was reached at 1.13 hr. The elimination half life after single dose was about 4 hr regardless of the route of administration. Nomifensine was extensively distributed in body fluids and tissues, with an apparent volume of distribution of 8.69 L/kg. The AUC of free nomifensine after oral dosing was only 26.5% of that after intravenous infusion. Absorption from the gastrointestinal tract was complete, and the AUCs of total nomifensine were equal after all treatments. The AUC of free nomifensine decreased substantially and the elimination half life was shortened after a 2-wk dosing period. It was concluded that the main reason for limited bioavailability seems to be extensive first-pass metabolism during the absorption process and that a marked induction of the metabolizing enzymes is suggested; therefore, an increase in nomifensine dosage may be needed in some patients to maintain a full therapeutic effect. /Nomifensine maleate/ The pharmacokinetics of nomifensine were assessed in 12 healthy men and women following single 100 mg oral doses of nomifensine maleate (Merital). Mean plasma half-life of unconjugated nomifensine was 1.9 hr and for total nomifensine was 4.1 hr. The mean peak plasma concentration of unconjugated nomifensine was 130 + or - 36.5 ug/L for men and 38 + or - 9.7 ug/L for women, a significant difference. No differences between males and females were observed for peak total nomifensine plasma concentrations. ... Oral clearance was higher in women than men as were the respective volumes of distribution. The disposition of radioactivity from (14)C-nomifensine has been compared in pregnant and non-pregnant female rats by examining plasma profiles, the qualitative tissue distribution (whole body autoradiography) and the quantitative tissue distribution of radioactivity. The clearance of radioactivity of (14)C-nomifensine from the plasma of pregnant and non-pregnant rats was similar and was complex with secondary peaks and plateaux after both oral and intravenous dosing. Maximum plasma levels (mean +/- S.D., 0.20 +/- 0.05 and 0.22 +/- 0.02 ug equivalents nomifensine/mL plasma for pregnant and non-pregnant rats respectively) occurred at 30 to 45 min after oral dosing. The biological half-life of radioactivity in plasma was between 4 and 5 hr for both routes of administration, although there was an additional rapid initial phase (half life approx. 20 min) after intravenous dosing. Whole body autoradiography also showed a very similar tissue distribution pattern of radioactivity between pregnant and non-pregnant rats with extensive distribution from blood into tissue. Only traces of radioactivity from (14)C-nomifensine were seen to cross the placenta into the fetuses of 15-day pregnant rats and these rapidly cleared with time. Slightly higher amounts were seen to cross the fetuses of 18-day pregnant rats and radioactivity was seen in the fetal brain, heart, liver and lung. Quantitative tissue distribution studies confirmed these qualitative findings. The biological half-life of radioactivity in both adult and fetal tissues was approximately 5 hr, except for adult livers where a longer half-life of radioactivity of approximately 10 hr was found. Nomifensine (1 and 5 mg/kg) was administered to dogs orally and intravenously. The pharmacokinetics of the drug was evaluated. Nomifensine was rapidly absorbed from the gastro-intestinal tract reaching maximum concentration at 0.5-1 hr. The peak levels were directly proportional to the doses administered. The elimination half-life was 6 hr and only very small amounts were found in blood at 24 hr after administration. The apparent volume of distribution (Vd) was 120-149 1, suggesting an extensive distribution of the drug throughout body fluids and tissues. The area under the serum concentration-time curve (AUC) obtained after oral administration was significantly smaller than that after intravenous administration indicating incomplete bioavailability of the drug in oral form. The conjugation of nomifensine after the two different administration routes was also studied: the conjugation reaction was in equilibrium at 15 min after oral administration, while after intravenous administration, equilibrium was not reached until 1-1.5 hr. The metabolism of nomifensine occurred in the gastrointestinal membranes and or in the liver during the absorption process; the first-pass effect was marked. Nomifensine is rapidly absorbed and is widely distributed. After oral administration, peak levels are obtained within one to two hours. It has a rapid elimination half life of two hours and is primarily excreted in the urine, 60% to 65% unchanged, the remainder as metabolites. Nomifensine is excreted in breast milk. Despite the short half life, electroencephalographic studies in humans reveal that maximal central nervous system effects are sustained up to eight hours after the oral administration of 75 or 150 mg of nomifensine. Thus, central effects persist long after the plasma levels of the drug have diminished. Metabolism / Metabolites The metabolism of nomifensine maleate was studied in 6 healthy subjects aged 22-41 yr, after single oral administration of two 50 mg capsules and 100 mg intravenous injection, and after 2 wk of oral daily administration of 150 mg in capsule form. The determination of the 3 main metabolites of nomifensine maleate in plasma and urine was performed by HPLC. The 3 principal metabolites reached maximum plasma concentrations rapidly (1-1.5 hr), less than 10% as a free, unconjugated form and were eliminated rapidly (elimination half life between 6.8 and 9.0 hr). Only very low concentrations of free metabolites were found in plasma after 24 hr. Two wk of dosing had no significant influence on the elimination of half life or AUC values of the metabolites, indicating no change in the hydroxylation and methylation reactions and there were no changes in the conjugation reactions. Nomifensine had a very short half life and no tendency for accumulation after repeated doses. It was concluded that the clinical pharmacokinetic profile of nomifensine maleate is not significantly changed by the kinetic behavior of its 3 main metabolites after the usual maintenance doses. /Nomifensine maleate/ Nomifensine is an antidepressant agent that was removed from use because of a high incidence of hemolytic anemia. It contains an N-methyl-8-aminotetrahydroisoquinoline ring which has the potential to be oxidized to quaternary dihydroisoquinolinium and isoquinolinium ions, albeit such a transformation had not been previously observed. In this report, ... the conversion of nomifensine to a dihydroisoquinolinium ion metabolite by several human enzymes /is demonstrated/. Human liver microsomes supplemented with NADPH generated the dihydroisoquinolinium ion metabolite along with other hydroxylated metabolites, whereas when supplemented with t-butyl peroxide, only the dihydroisoquinolinium ion metabolite was observed. Monoamine oxidase A, but not monoamine oxidase B, catalyzed this reaction, as well as human hemoglobin supplemented with H2O2. Human myeloperoxidase catalyzed this reaction in the presence of H2O2, and activation of the reaction was observed when incubations were conducted in the presence of acetaminophen at concentrations relevant to those measured in humans. The reaction was also observed in human whole blood. The equilibrium between the dihydroisoquinolinium ion and carbinolamine was shown to have a pK of about 11.7. Biological Half-Life The pharmacokinetics of nomifensine were studied in 6 subjects aged 23-41 yr, after single oral administration of two 50 mg capsules of nomifensine maleate (Nomival) and 100 mg intravenous injection, and after 2 wk of oral daily administration of 150 mg in capsule form. ... The elimination half life after single dose was about 4 hr regardless of the route of administration. ... /Nomifensine maleate/ The pharmacokinetics of nomifensine were assessed in 12 healthy men and women following single 100 mg oral doses of nomifensine maleate (Merital). Mean plasma half-life of unconjugated nomifensine was 1.9 hr and for total nomifensine was 4.1 hr. The disposition of radioactivity from (14)C-nomifensine has been compared in pregnant and non-pregnant female rats ... . The biological half-life of radioactivity in plasma was between 4 and 5 hr for both routes of administration /oral and intravenous/, although there was an additional rapid initial phase (half life approx. 20 min) after intravenous dosing. ... The biological half-life of radioactivity in both adult and fetal tissues was approximately 5 hr, except for adult livers where a longer half-life of radioactivity of approximately 10 hr was found. Nomifensine (1 and 5 mg/kg) was administered to dogs orally and intravenously. ... The elimination half-life was 6 hr and only very small amounts were found in blood at 24 hr after administration. ... |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
Six epileptic patients (aged 21-28 yr) on either phenytoin and phenobarbital (phenobarbitone) or phenytoin and carbamazepine, and 6 controls (aged 21-25 yr), were given single oral doses of 30 mg mianserin and 100 mg of nomifensine, at least one month apart, to determine if treatment with anticonvulsants would influence the pharmacokinetics of mianserin and nomifensine. The results showed that plasma levels of mianserin and nomifensine were significantly reduced in epileptic patients treated with anticonvulsants. Non-Human Toxicity Values LD50 rat intravenous 72 mg/kg LD50 mouse oral 260 mg/kg LD50 mouse intravenous 90 mg/kg LD50 guinea pig intravenous 264 mg/kg |
| 其他信息 |
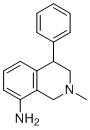
Nomifensine is an N-methylated tetrahydroisoquinoline carrying phenyl and amino substituents at positions C-4 and C-8, respectively. It has a role as a dopamine uptake inhibitor.
Nomifensine, formerly marketed as Merital capsules, was associated with an increased incidence of hemolytic anemia. The approved application holder removed Merital capsules from the market on January 23, 1986. FDA published a notice of its determination that Merital capsules were removed from the market for safety reasons (see the Federal Register of June 17, 1986 (51 FR 21981)). Approval of the NDA for Merital capsules was withdrawn on March 20, 1992 (see the Federal Register of March 20, 1992 (57 FR 9729)). Also withdrawn from the Canadian and UK markets. An isoquinoline derivative that prevents dopamine reuptake into synaptosomes. The maleate was formerly used in the treatment of depression. It was withdrawn worldwide in 1986 due to the risk of acute hemolytic anemia with intravascular hemolysis resulting from its use. In some cases, renal failure also developed. (From Martindale, The Extra Pharmacopoeia, 30th ed, p266) Mechanism of Action Nomifensine ... while it resembles the imipramine type agents in many of the pharmacologic tests used in screening potential antidepressive activity, nomifensine is distinct in its potent inhibitory effects on the neuronal reuptake of dopamine. ... Nomifensine's dopaminergic profile is essentially restricted to reuptake blockade. It has no presynaptic effects on dopamine release as would amphetamine and no post synaptic effects on adenylate-cyclase linked dopamine (D-1) receptors as would apomorphine (a dopamine agonist). In addition to its potent dopaminergic effects, nomifensine has important effects on the noradrenergic system. Nomifensine is more than 20 times as potent as imipramine, and equipotent to desipramine, in inhibiting the neuronal reuptake of norepinephrine. In electrophysiologic studies nomifensine is four times as active, within the locus coeruleus (a major noradrenergic brain nucleus), as desipramine, and nomifensine is 20 times as potent as imipramine with this test system. In accord with such activity, chronic treatment with nomifensine leads to a reduction in the sensitivity of postsynaptic beta-noradrenergic receptors. Nomifensine has relatively weak alpha-adrenergic blocking effect. In vitro binding studies suggest that nomifensine is six times less potent than imipramine at antagonism of the alpha-1 receptor, but twice as potent at the alpha-2. In this regard, nomifensine has less alpha-blocking activity than trazodone. Based upon these characteristics, both sedation and adverse cardiovascular (eg, hypotensive) effects should not be prominent with nomifensine. Effects on the serotonergic system are varied. Nomifensine is a weak inhibitor of serotonin reuptake into rat brain synaptosomes, and is 1/300 times as potent as imipramine, and equipotent to desipramine, in this regard. This is confirmed in electrophysiologic studies where nomifensine has extremely weak, although not absent, activity within the dorsal raphe. However, while nomifensine appears to be inactive in serotonergic systems it has some binding affinity for serotonergic receptors; it is as avid as imipramine for the 5-HT 1 receptor, but is less than 1/100 as avid as imipramine for the 5-HT 2 receptor. For more Mechanism of Action (Complete) data for NOMIFENSINE (6 total), please visit the HSDB record page. Therapeutic Uses Nomifensine, formerly marketed as Merital capsules, was associated with an increased incidence of hemolytic anemia. The approved application holder removed Merital capsules from the market on January 23, 1986. FDA published a notice of its determination that Merital capsules were removed from the market for safety reasons (see the Federal Register of June 17, 1986 (51 FR 21981)). Approval of the NDA for Merital capsules was withdrawn on March 20, 1992 (see the Federal Register of March 20, 1992 (57 FR 9729)). Also withdrawn from the Canadian and UK markets. Nomifensine, introduced in 1985, is a tetrahydroisoquinoline antidepressant. /No longer approved for use in the US/ Drug Warnings Nomifensine, formerly marketed as Merital capsules, was associated with an increased incidence of hemolytic anemia. The approved application holder removed Merital capsules from the market on January 23, 1986. FDA published a notice of its determination that Merital capsules were removed from the market for safety reasons (see the Federal Register of June 17, 1986 (51 FR 21981)). Approval of the NDA for Merital capsules was withdrawn on March 20, 1992 (see the Federal Register of March 20, 1992 (57 FR 9729)). Also withdrawn from the Canadian and UK markets. The development of sudden fever in 6 women and one man (mean age, 64 yr) during oral nomifensine therapy at usual doses is reported. During original therapy, fever occurred within 2 wk (range, a few hr to 30 days) and sooner during a second treatment (one to 3 days). In 6 cases, the recurrence of the fever closely following the rechallenge is suggestive of a causal relationship and implies a hypersensitivity reaction. In humans, nomifensine has been found to mildly elevate heart rate but not to affect orthostatic blood pressure, QRS width, QT interval, or the His-bundle electrocardiogram at doses up to 200 mg/day for three weeks. ... Potent dopaminergic activity may induce, or bring out, dyskinetic movements in susceptible individuals as has been described in at least one case. For more Drug Warnings (Complete) data for NOMIFENSINE (6 total), please visit the HSDB record page. Pharmacodynamics Nomifensine is a dopamine reuptake inhibitor test-marketed in the United States by Hoechst AG (now Novartis) that increases the amount of synaptic dopamine available to receptors by blocking dopamine's re-uptake transporter. Nomifensine is now mainly used in scientific research, particularly in studies involving dopamine release in response to addiction. |
| 分子式 |
C16H18N2
|
|---|---|
| 分子量 |
238.334
|
| 精确质量 |
238.147
|
| 元素分析 |
C, 80.63; H, 7.61; N, 11.75
|
| CAS号 |
24526-64-5
|
| 相关CAS号 |
Nomifensine maleate; 32795-47-4; Nomifensine-d3 maleate; 1795140-41-8
|
| PubChem CID |
4528
|
| 外观&性状 |
Solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
378.4±42.0 °C at 760 mmHg
|
| 熔点 |
179-181°
|
| 闪点 |
164.0±23.0 °C
|
| 蒸汽压 |
0.0±0.9 mmHg at 25°C
|
| 折射率 |
1.623
|
| LogP |
2.15
|
| tPSA |
29.26
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
18
|
| 分子复杂度/Complexity |
272
|
| 定义原子立体中心数目 |
0
|
| SMILES |
NC1=CC=CC2=C1CN(C)CC2C3=CC=CC=C3
|
| InChi Key |
XXPANQJNYNUNES-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C16H18N2/c1-18-10-14(12-6-3-2-4-7-12)13-8-5-9-16(17)15(13)11-18/h2-9,14H,10-11,17H2,1H3
|
| 化学名 |
2-methyl-4-phenyl-3,4-dihydro-1H-isoquinolin-8-amine
|
| 别名 |
HSDB7702; CCRIS9179; HSDB-7702; CCRIS-9179; Nomifensine
|
| HS Tariff Code |
2934.99.03.00
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ≥ 100 mg/mL (~419.6 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (10.49 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (10.49 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.1959 mL | 20.9793 mL | 41.9586 mL | |
| 5 mM | 0.8392 mL | 4.1959 mL | 8.3917 mL | |
| 10 mM | 0.4196 mL | 2.0979 mL | 4.1959 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。