| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
Antioxidant; anti-inflammatory
|
|---|---|
| 体外研究 (In Vitro) |
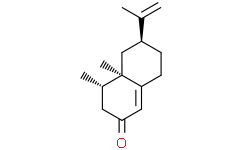
(+)-nootkatone是一种倍半萜,是4,4a,5,6,7,8-六氢萘-2(3H)-酮,在4和4a位被甲基取代,在6位被异丙烯基取代(4R,4aS,6R立体异构体)。它具有植物代谢产物、香味和驱虫剂的作用。它是一种倍半萜、烯酮和碳双环化合物。
|
| 体内研究 (In Vivo) |
在包括 Morris 水迷宫和 Y 迷宫在内的行为测试中,Nootkatone (10 mg/kg) 组表现良好。根据组织学检查和免疫组织化学分析的结果,Nootkatone (10 mg/kg) 逆转了 LPS 诱导的神经元变性和星形胶质细胞活化的影响,特别是在海马体中。 ELISA (10 mg/kg) 降低了这些炎症细胞因子升高的表达[1]。
|
| 动物实验 |
Neuroinflammatory responses play a crucial role in the pathogenesis of Alzheimer's disease (AD). Our previous study demonstrated that petroleum ether extracts from Alpiniae Oxyphyllae Fructus(AOF) could attenuate lipopolysaccharide (LPS)-induced learning and memory impairment in mice, which could be associated with its inhibitory effect on neuroinflammation. Therefore, our present study is to investigate the potential therapeutic neuroprotective effects of nootkatone (NKT) on an AD mouse model induced by intracerebroventricular injection of LPS. We found that NKT (10 mg/kg) group showed good performance in behavior experiments including Y-maze test and Morris water maze test. The results of histopathological examination and immunohistochemical analysis showed that LPS induced degeneration of neurons and activation of microglia particularly in hippocampus and NKT (10 mg/kg) reversed these changes. Enzyme linked immunosorbent assay and western blot analysis also demonstrated that the model group had increased expression of IL-1β, IL-6, TNF-α, NLRP3 and NF-κB p65, especially in hippocampus relative to sham-operated group, and NKT (10 mg/kg) decreased the high expression of these inflammatory cytokines. Collectively, these data indicated that LPS-induced learning and memory impairments in mice could be improved by NKT, which was associated with attenuating neuroinflammatory responses. Our study indicated that NKT could act as a potential therapeutic agent for the treatment of neuroinflammation and AD.[1]
|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Nootkatone forms crystals; however, commercial product is a colorless to yellowish liquid. It is used in beverages to impart grapefruit flavor; in fragrance compositions with other citrus oils. Potential bio-based pesticide to kill ants, termites, mosquitoes, cockroaches, and ticks, including Ixodes scapularis (blacklegged tick), whose bite can transmit bacteria that cause Lyme disease in humans and other animals. HUMAN EXPOSURE AND TOXICITY: No human data were found. ANIMAL STUDIES: In a 28-day study, rats (male and female) were given nootkatone at a dose of 10 mg/kg bw per day by oral gavage. No clinically observable signs of toxicity were reported. Histopathological examination revealed accumulation of globular eosinophilic material in the tubular epithelium of male rats treated with nootkatone at a dose of 10 mg/kg bw per day. This finding is consistent with the presence of hyaline droplet nephropathy, which results from the excessive accumulation of alpha-2u-globulin in renal proximal tubular epithelial cells. Accumulation of alpha-2u-globulin in the proximal tubular epithelium is considered to be of no relevance to humans. ECOTOXICITY STUDIES: Encapsulation of nootkatone improved toxicity for tick control, reduced nootkatone volatility, and reduced plant phytotoxicity. Antidote and Emergency Treatment /SRP:/ Immediate first aid: Ensure that adequate decontamination has been carried out. If patient is not breathing, start artificial respiration, preferably with a demand valve resuscitator, bag-valve-mask device, or pocket mask, as trained. Perform CPR if necessary. Immediately flush contaminated eyes with gently flowing water. Do not induce vomiting. If vomiting occurs, lean patient forward or place on the left side (head-down position, if possible) to maintain an open airway and prevent aspiration. Keep patient quiet and maintain normal body temperature. Obtain medical attention. /Poisons A and B/ /SRP:/ Basic treatment: Establish a patent airway (oropharyngeal or nasopharyngeal airway, if needed). Suction if necessary. Watch for signs of respiratory insufficiency and assist ventilations if needed. Administer oxygen by nonrebreather mask at 10 to 15 L/min. Monitor for pulmonary edema and treat if necessary ... . Monitor for shock and treat if necessary ... . Anticipate seizures and treat if necessary ... . For eye contamination, flush eyes immediately with water. Irrigate each eye continuously with 0.9% saline (NS) during transport ... . Do not use emetics. For ingestion, rinse mouth and administer 5 mL/kg up to 200 mL of water for dilution if the patient can swallow, has a strong gag reflex, and does not drool ... . Cover skin burns with dry sterile dressings after decontamination ... . /Poisons A and B/ /SRP:/ Advanced treatment: Consider orotracheal or nasotracheal intubation for airway control in the patient who is unconscious, has severe pulmonary edema, or is in severe respiratory distress. Positive-pressure ventilation techniques with a bag valve mask device may be beneficial. Consider drug therapy for pulmonary edema ... . Consider administering a beta agonist such as albuterol for severe bronchospasm ... . Monitor cardiac rhythm and treat arrhythmias as necessary ... . Start IV administration of D5W TKO /SRP: "To keep open", minimal flow rate/. Use 0.9% saline (NS) or lactated Ringer's (LR) if signs of hypovolemia are present. For hypotension with signs of hypovolemia, administer fluid cautiously. Watch for signs of fluid overload ... . Treat seizures with diazepam or lorazepam ... . Use proparacaine hydrochloride to assist eye irrigation ... . /Poisons A and B/ |
| 参考文献 | |
| 其他信息 |
(+)-nootkatone is a sesquiterpenoid that is 4,4a,5,6,7,8-hexahydronaphthalen-2(3H)-one which is substituted by methyl groups at positions 4 and 4a, and by an isopropenyl group at position 6 (the 4R,4aS,6R stereoisomer). It has a role as a plant metabolite, a fragrance and an insect repellent. It is a sesquiterpenoid, an enone and a carbobicyclic compound.
Nootkatone has been reported in Mandragora autumnalis, Citrus reticulata, and other organisms with data available. |
| 分子式 |
C15H22O
|
|---|---|
| 分子量 |
218.3346
|
| 精确质量 |
218.167
|
| CAS号 |
4674-50-4
|
| PubChem CID |
1268142
|
| 外观&性状 |
White to yellow solid
|
| 密度 |
1.0±0.1 g/cm3
|
| 沸点 |
318.6±42.0 °C at 760 mmHg
|
| 熔点 |
35-39ºC
|
| 闪点 |
142.1±18.7 °C
|
| 蒸汽压 |
0.0±0.7 mmHg at 25°C
|
| 折射率 |
1.503
|
| LogP |
3.84
|
| tPSA |
17.07
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
16
|
| 分子复杂度/Complexity |
364
|
| 定义原子立体中心数目 |
3
|
| SMILES |
O=C1C([H])=C2C([H])([H])C([H])([H])[C@@]([H])(C(=C([H])[H])C([H])([H])[H])C([H])([H])[C@@]2(C([H])([H])[H])[C@]([H])(C([H])([H])[H])C1([H])[H]
|
| InChi Key |
WTOYNNBCKUYIKC-JMSVASOKSA-N
|
| InChi Code |
InChI=1S/C15H22O/c1-10(2)12-5-6-13-8-14(16)7-11(3)15(13,4)9-12/h8,11-12H,1,5-7,9H2,2-4H3/t11-,12-,15+/m1/s1
|
| 化学名 |
(4R,4aS,6R)-4,4a-dimethyl-6-prop-1-en-2-yl-3,4,5,6,7,8-hexahydronaphthalen-2-one
|
| 别名 |
NOOTKATONE; (+)-Nootkatone; 4674-50-4; Nootkanone; (+/-)-Nootkatone; Nootkatone, (+/-)-; Nootkatone (+/-)-form [MI]; UNII-3K3OKV2A5A;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~458.02 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (11.45 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (11.45 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (11.45 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.5802 mL | 22.9011 mL | 45.8022 mL | |
| 5 mM | 0.9160 mL | 4.5802 mL | 9.1604 mL | |
| 10 mM | 0.4580 mL | 2.2901 mL | 4.5802 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。