| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
Sodium-dependent bile acid transporter (ASBT)
|
|---|---|
| 体外研究 (In Vitro) |
胆汁酸稳态对人类的许多代谢和免疫功能至关重要。胆汁酸的肠肝循环非常有效,约95%的肠胆汁酸被重新吸收。干扰肠道胆汁酸摄取预计会严重影响肠道和全身胆汁酸水平。在这里,我们的目的是预测顶端钠依赖性胆汁酸转运蛋白(ASBT)抑制对全身血浆水平的影响。为此,我们将体外Caco-2细胞转运测定与基于生理学的(PBK)建模相结合。我们使用选择性ASBT抑制剂odevixibat (ODE)作为模型化合物。在培养插入物上生长的Caco-2细胞用于获得甘胆酸(GCA)的转运动力学参数。测定GCA的表观米氏常数(Km,app)、表观最大肠道转运速率(Vmax,app)和ODE抑制常数(Ki)。这些动力学参数被纳入PBK模型,用于预测ASBT对血浆胆汁酸水平的抑制作用。GCA以活性和钠依赖的方式在Caco-2细胞上运输,表明存在功能性ASBTodevixibat(ODE)剂量依赖性地抑制GCA转运。PBK模型预测,口服剂量的ODE降低了血浆中的结合胆汁酸水平。我们的模拟与体内数据相匹配,为胆汁酸-PBK模型中活性肠道胆汁酸摄取的结合提供了第一个原理证明。这种方法将来可用于预测其他ASBT抑制剂对血浆和肠道胆汁酸水平的影响[2]。
|
| 体内研究 (In Vivo) |
Odevixibat (A4250)(饲料中 0.01%(w/w);4 周)可改善硬化性胆管炎,显着降低血清 BA、碱性磷酸酶和丙氨酸转氨酶水平,以及促炎和促纤维化基因的表达Mdr2-/-小鼠的肝脏和胆管增殖[1]。此外,在刺激 Ntcp 和 Cyp7a1 的同时,Odevixibat (A4250) 显着降低胆汁通量和胆汁 BA 输出,这与 bsep 转录减少相一致 [1]。
Odevixibat(A4250)改善了Mdr2−/-小鼠的硬化性胆管炎,并显著降低了血清丙氨酸氨基转移酶、碱性磷酸酶和BAs水平、促炎(Tnf-α、Vcam1、Mcp-1)和促纤维化(Col1a1、Col1a2)基因的肝脏表达以及胆管增殖(细胞角蛋白19(CK19)的mRNA和免疫组织化学)。此外,Odevixibat (A4250)显著降低了胆汁流量和胆汁BA输出,这与Bsep转录减少有关,同时诱导了Ntcp和Cyp7a1。重要的是,A4250显著减少了胆汁中BA的分泌,但保留了HCO3-和胆汁磷脂的分泌,导致HCO3-/BA和PL/BA比值增加。此外,A4250显著增加了粪便中BA的排泄,而不会引起腹泻,并改变了BA池的组成,导致初级BA牛磺胆酸和牛磺胆酸的浓度降低。[1] 选择性ASBT抑制剂Odevixibat (A4250)可改善Mdr2−/-小鼠的胆汁淤积性肝脏和胆管损伤[1] Odevixibat (A4250)在Mdr2−/-小鼠中具有良好的耐受性,在短期或长期喂养后(分别为1周和4周;数据未显示)对动物行为或体重没有影响。在我们的研究中,我们重点研究了8周龄Mdr2−/-小鼠胆汁淤积性肝脏和胆道损伤的潜在变化,这是这些小鼠胆管损伤完全建立并出现明显胆汁淤积的时间点。根据肝脏组织学测定,喂食4周后,A4250改善了Mdr2−/-小鼠的胆管损伤,包括胆管炎和洋葱皮型纤维化(图1A)。值得注意的是,作为肝细胞损伤标志物的血清ALT在2周后已经显著降低(图1B),而在Mdr2−/-小鼠中喂食A4250 4周后,胆汁淤积的血清标志物(AP和BA)显著降低(见图1C和D)。这表明需要更长的治疗时间来观察对胆道损伤的全面影响。与血清肝酶和BA一致,喂食A4250后,Mdr2−/-小鼠的血清胆红素也略有但显著降低(0.085±0.0 vs.0.16±0.1 mg/dl)。有趣的是,补充A4250后,Mdr2−/-小鼠的血清甘油三酯水平保持不变,而血清胆固醇升高(补充图1)。与组织学和生化结果一致,A4250治疗后Mdr2−/-小鼠的肝脏LW/BW和SW/BW%比值显著降低(图1E),反映了其对肝损伤的总体有益作用。此外,通过CK19-IHC染色和定量(图2B)以及mRNA表达评估(图2C)确定,A4250显著降低了胆管的增殖(图2A)。总之,这些发现证实了ASBT抑制对硬化性胆管炎小鼠模型中胆汁淤积性肝脏和胆管损伤的有益作用。 Odevixibat (A4250)可减轻Mdr2−/-小鼠的肝脏炎症和导管周围纤维化[1] 由于胆道疾病中胆管的持续炎症和反应性增殖与胆管纤维化的发展有关[3]。接下来,我们测量了Tnf-α、Mcp-1和Vcam-1的mRNA表达,这些细胞因子是参与Mdr2−/-小鼠肝损伤发病机制的主要促炎细胞因子(图3A),此外还测量了胆汁纤维化标志物如Col1a1和Col1a2的表达(图3B)。有趣的是,在接受Odevixibat (A4250)治疗的小鼠中,Col1a1和Col1a2等促纤维化基因的转录显著降低(图3B)。相反,两组之间的肝脏羟脯氨酸含量和α-SMA蛋白水平没有差异(图3C、3D)。与促炎细胞因子和促纤维化基因的表达受到抑制一致,天狼星红染色的定量显示,胆周纤维化显著减少,总体纤维化有减少的趋势(图3E,F)。综上所述,这些数据表明,在Mdr2−/-小鼠的4周治疗过程中,A4250具有抗炎和中度抗纤维化作用。 奥地昔布(A4250)显著改变Mdr2−/-小鼠的胆汁酸稳态[1] 重要的是,在喂食Odevixibat (A4250)后,Mdr2−/-小鼠回肠中Asbt以及基底外侧BA转运蛋白Ost-α和Ost-β的基因表达保持不变,而Fgf15的表达显著降低,Fgf15是BA合成的重要肠道调节因子[15]和细胞内BA传感器FXR的靶标(图4A),从而证实了回肠BA摄取的有效抑制。同样,A4250喂养导致肝脏中BA合成限速酶胆固醇7α羟化酶(Cyp7a1)和正弦BA摄取转运蛋白Ntcp的转录增强3倍,而小管BA输出泵Bsep的表达降低(图4B)。此外,A4250降低了BA解毒酶Cyp3a11、Ugt1a1和Ugt2b5以及正弦输出转运蛋白Mrp3的基因表达,而正弦输出蛋白Mrp4、Ost-α和Ost-β没有显著改变(图4B)。总的来说,这些发现反映了肝细胞BA负荷的降低和肝BA稳态的代偿性变化。 ASBT抑制降低胆汁BA输出并改变胆汁BA组成[1] Odevixibat(A4250)显著降低了胆汁流量(图5A),这与胆汁BA浓度和输出量的显著降低有关(图5B,C)。此外,Odevixibat (A4250)显著改变了胆汁中BA的组成,降低了原代BA牛磺胆酸(TβMCA)和牛磺胆酸的浓度(图5D)。相比之下,次级BAs牛磺脱氧胆酸(TDCA)和牛磺脱氧胆碱酸(THDCA)的浓度增加,而牛磺ω-muricholic酸(TωMCA)和牛粪脱氧胆酸(TUDCA)降低(图5E)。值得注意的是,在喂食食物或A4250的小鼠胆汁中没有发现未结合的BA。在喂食A4250的小鼠中,粪便总BA水平显著升高(图5F)。值得注意的是,粪便中没有发现表明存在硫酸化BAs的阴离子。重要的是,相比之下,A4250喂养和食物喂养的小鼠BA输出减少、胆汁浓度以及保护性HCO3-输出没有差异(图5B,C),而A4250喂养的Mdr2−/−小鼠的HCO3-和PL相对输出显著增加,这可以通过HCO3-/BA和PL/BA比率的增加来证明(图5B)。由于非胶束结合的游离BA被认为会引发Mdr2−/-小鼠的胆道疾病,胆汁BA输出的显著减少和HCO3−比例的同时增加可能是毒性胆汁介导的肝损伤的重要保护机制。 奥地昔布(A4250)治疗不会促进Mdr2−/-小鼠的腹泻或肠道炎症[1] 尽管粪便总BA水平显著升高(图5F),但通过评估粪便量和稠度,小鼠没有出现腹泻。因此,我们观察到对照组和Odevixibat(A4250)喂养的小鼠的粪便重量没有差异。(对照组小鼠每天粪便1.30克,而A4250喂养的小鼠每天粪便1.38克)。我们还探讨了A4250是否会促进结肠炎症。重要的是,常规组织学显示,在喂食食物和A4250的Mdr2−/-小鼠之间,结肠壁的结构和炎性细胞浸润没有差异(图6A)。此外,巨噬细胞标志物F4/80(数据未显示)和增殖标志物Ki67(图6B,E)的IHC染色显示,实验组之间没有显著差异。与这些发现一致,A4250治疗的Mdr2−/-小鼠回肠中促炎细胞因子Tnf-α的转录保持不变(图6D)。由于肠BA浓度增加时肠内分泌细胞释放的GLP-1可能对胆管细胞具有直接保护作用[18],我们接下来探讨了GLP-1介导的肠肝信号传导是否可能有助于A4250介导的胆汁淤积有益作用。值得注意的是,GLP-1 IHC在对照组和A4250喂养的动物之间没有显示出差异,而在A4250饲养的Mdr2−/-小鼠中,前胰高血糖素的回肠mRNA表达降低(图6C和F),表明这种机制不太可能有助于A4250介导的对胆汁淤积性肝脏和胆管损伤的保护。 奥地昔布(A4250)可减小胆囊大小[1] 由于胆汁稳态在胆囊生理中起着至关重要的作用,我们还研究了胆汁流量和胆汁BA输出减少对胆囊形态的影响。有趣的是,通过增加固有肌层厚度和粘膜褶皱数量,在Odevixibat(A4250)喂养的Mdr2−/-小鼠中,胆囊壁厚度增加(补充图2A,B)。重要的是,在固有层、固有肌层或上皮中没有检测到F4/80染色所反映的炎性细胞浸润的增加(补充图2C),从而排除了胆囊壁增厚的炎症介导机制。由于Fgf15通过抵消胆囊收缩素来刺激胆囊充盈[19],胆囊壁厚度的增加可能反映了Fgf15信号传导的减少,导致A4250喂养的Mdr2−/-小鼠胆囊收缩。重要的是,用A4250处理的Mdr2−/-小鼠的胆囊没有充满沉积物或结石。 |
| 细胞实验 |
胆汁酸调节消化和免疫功能。肠道内胆汁酸再摄取过少与多种疾病有关,包括炎症性肠病。本研究以药物odevixibat (ODE)/奥地昔布为例,研究了减少胆汁酸吸收如何影响人体胆汁酸水平odevixibat(ODE)通过阻断肠道细胞中的肠道胆汁酸转运蛋白来减少胆汁酸吸收。在有和没有ODE的情况下,测量了胆汁酸通过通常用于模拟肠屏障的肠细胞系的运输,并使用数学建模将实验室结果转化为全身效应。这种组合方法准确地预测了ODE对人类肠道和血液胆汁酸水平的已知影响。这种新方法可用于预测其他化学物质对肠道胆汁酸吸收以及肠道和血液胆汁酸水平的影响,而不是动物试验[2]。
|
| 动物实验 |
Animal/Disease Models: Eightweeks old Mdr2-/- (Abcb4-/-) mice (cholestatic liver injury and sclerosing cholangitis model) [1]
Doses: 0.01% (w/w) in feed Administration time: 4-week Experimental Results: Cholestasis diminished in mouse liver and bile duct injury model. Odevixibat (A4250) (a specific ASBT inhibitor) was synthesized. Eight week old male Mdr2−/− mice received either control diet or a diet supplemented with 0.01% (w/w) A4250 either for 4 weeks or for 1 week. The 4 week treatment protocol was used for biochemical, molecular and histological data analysis, whereas the 1 week treated mice were subjected to bile flow measurement. [1] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
A 7.2 mg single oral dose of odevixibat in adults reaches a Cmax of 0.47 ng/mL, with an AUC0-24h of 2.19 h\*ng/mL. The majority of adult and pediatric patients, given a therapeutic dose, do not have detectable plasma concentrations of odevixibat. Odevixibat is 82.9% recovered in the feces and <0.002% recovered in the urine. The dose recovered in the feces is 97% unchanged parent compound. The majority of adult and pediatric patients, given a therapeutic dose, do not have detectable plasma concentrations of odevixibat. Therefore, a volume of distribution has not been calculated. The majority of adult and pediatric patients, given a therapeutic dose, do not have detectable plasma concentrations of odevixibat. Therefore, the clearance has not been calculated. Metabolism / Metabolites Odevixibat is largely unmetabolized, however a small amount is metabolized _in vitro_ by mono-hydroxylation. The exact structure of the metabolite has not been characterized as a primary endpoint of the clinical trial was to characterize the structure of metabolites accounting for >10% of the dose in plasma, urine, or feces. No metabolites have been identified at such a high concentration. Biological Half-Life A 7.2 mg oral dose of odevixibat has a mean half life of 2.36 hours in adults. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In trials of odevixibat in children with cholestatic liver diseases, serum ALT elevations of greater than 3 times ULN arose in 8% to 11% of treated participants and were particularly common with long term therapy. However, children with PFIC typically have serum ALT and AST elevations, and it was difficult to establish whether mild-to-moderate serum enzyme elevations were due to odevixibat therapy or to the spontaneous fluctuations that occur with the underlying disease. Clinically apparent liver injury with jaundice or hepatic decompensation has not been reported in children treated with odevixibat, although the total clinical experience with its use is limited. Likelihood score: E* (suspected but unproven cause of clinically apparent liver injury). Protein Binding Due to the low systemic abosrption of odevixibat, plasma protein binding studies could not be performed _in vivo_. Odevixibat is >99% protein bound _in vitro_. |
| 参考文献 |
|
| 其他信息 |
Odevixibat, or A4250, is an ileal sodium/bile acid cotransporter inhibitor indicated for the treatment of pruritus in patients older than 3 months, with progressive familial intrahepatic cholestasis (PFIC). Odevixibat is the first approved non-surgical treatment option for PFIC. Previous therapies for PFIC included a bile acid sequestrant such as [ursodeoxycholic acid]. Odevixibat was granted FDA and Health Canada approval on 20 July 2021 and 13 November 2023 respectively.
Odevixibat is an Ileal Bile Acid Transporter Inhibitor. The mechanism of action of odevixibat is as an Ileal Bile Acid Transporter Inhibitor. Odevixibat is an orally available inhibitor of the ilieal bile salt transporter which is used to treat severe pruritus in patients with cholestatic liver disease such as progressive familial intrahepatic cholestasis. Odevixibat is associated with transient serum enzyme elevations particularly with long term therapy but has not been linked to instances of clinically apparent liver injury with jaundice, although experience with its use has been limited. Drug Indication Odevixibat is indicated for the treatment of pruritus in patients older than 3 months and 6 months with progressive familial intrahepatic cholestasis (PFIC) by the FDA and Health Canada respectively. It is also indicated for the treatment of cholestatic pruritus in patients 12 months of age and older with Alagille Syndrome. Odevixibat may not be effective in patients with PFIC type 2 with ABCB11 variants since these patients lack a functional bile salt export pump. Bylvay is indicated for the treatment of progressive familial intrahepatic cholestasis (PFIC) in patients aged 6 months or older (see sections 4. 4 and 5. 1). Treatment of Alagille syndrome Treatment of Progressive Familial Intrahepatic Cholestasis Treatment of biliary atresia Mechanism of Action Progressive familiar intrahepatic cholestasis (PFIC) is a group of autosomal recessive disorders leading to cholestasis, fibrosis, and eventually a need for liver transplantation. Patients with PFIC require liver transplants or develop hepatocellular carcinomas in their first few years of life. Many of these patients experience severe pruritus. The exact mechanism of pruritus is PFIC is not known, but lower concentrations of bile acids have been shown to reduce pruritus. Patients with certain forms of PFIC type 2, associated with a non-functional or absent bile salt export pump, are not expected to benefit from odevixibat treatment. The ileal sodium/bile acid cotransporter is a transport glycoprotein responsible for reabsorption of 95% of bile acids in the distal ileum. Odevixibat is a reversible inhibitor of the ileal sodium/bile acid contransporter. Patients taking odevixibat for a week experienced a 56% reduction in bile acid area under the curve with a 3 mg once daily dose. A 1.5 mg daily dose lead to a 43% reduction in bile acid area under the curve. The decreased reabsorption of bile acids, leads to reduced stimulation of FXR, which reduces expression of FGF19, reducing binding of FGF19 to FGF4R, decreasing inhibition of bile acid synthesis. Further synthesis of bile acids that will not be reabsorbed in the intestine contributes to lowering low density lipoprotein levels. Pharmacodynamics Odevixibat, or A4250, is an ileal sodium/bile acid cotransporter inhibitor indicated for the treatment of pruritus in patients older than 3 months, with progressive familiar intrahepatic cholestasis (PFIC). It has a moderate duration of action as it is given once daily. Odevixibat has a wide therapeutic index as patients were given single doses up to 10 mg while the maximum therapeutic dose is 6 mg daily. Patients should be counselled regarding the risks of elevated liver function tests, diarrhea, and fat soluble vitamin defiencies. Background and Aims Approximately 95% of bile acids (BAs) excreted into bile are reabsorbed in the gut and circulate back to the liver for further biliary secretion. Therefore, pharmacological inhibition of the ileal apical sodium-dependent BA transporter (ASBT/SLC10A2) may protect against BA-mediated cholestatic liver and bile duct injury. Methods Eight week old Mdr2−/− (Abcb4−/−) mice (model of cholestatic liver injury and sclerosing cholangitis) received either a diet supplemented with A4250 (0.01% w/w) – a highly potent and selective ASBT inhibitor – or a chow diet. Liver injury was assessed biochemically and histologically after 4 weeks of A4250 treatment. Expression profiles of genes involved in BA homeostasis, inflammation and fibrosis were assessed via RT-PCR from liver and ileum homogenates. Intestinal inflammation was assessed by RNA expression profiling and immunohistochemistry. Bile flow and composition, as well as biliary and fecal BA profiles were analyzed after 1 week of ASBT inhibitor feeding. Results A4250 improved sclerosing cholangitis in Mdr2−/− mice and significantly reduced serum alanine aminotransferase, alkaline phosphatase and BAs levels, hepatic expression of pro-inflammatory (Tnf-α, Vcam1, Mcp-1) and pro-fibrogenic (Col1a1, Col1a2) genes and bile duct proliferation (mRNA and immunohistochemistry for cytokeratin 19 (CK19)). Furthermore, A4250 significantly reduced bile flow and biliary BA output, which correlated with reduced Bsep transcription, while Ntcp and Cyp7a1 were induced. Importantly A4250 significantly reduced biliary BA secretion but preserved HCO3− and biliary phospholipid secretion resulting in an increased HCO3−/BA and PL/BA ratio. In addition, A4250 profoundly increased fecal BA excretion without causing diarrhea and altered BA pool composition, resulting in diminished concentrations of primary BAs tauro-β-muricholic acid and taurocholic acid.[1] |
| 分子式 |
C37H48N4O8S2
|
|---|---|
| 分子量 |
740.93
|
| 精确质量 |
740.291
|
| 元素分析 |
C, 59.98; H, 6.53; N, 7.56; O, 17.27; S, 8.65
|
| CAS号 |
501692-44-0
|
| 相关CAS号 |
501692-44-0;2409081-01-0 (hydrate);Odevixibat HCl;
|
| PubChem CID |
10153627
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 折射率 |
1.643
|
| LogP |
7.03
|
| tPSA |
208
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
17
|
| 重原子数目 |
51
|
| 分子复杂度/Complexity |
1230
|
| 定义原子立体中心数目 |
2
|
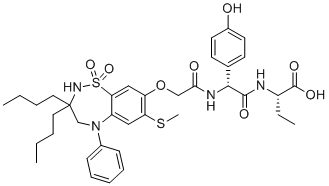
| SMILES |
CCCCC1(CN(C2=CC(=C(C=C2S(=O)(=O)N1)OCC(=O)N[C@H](C3=CC=C(C=C3)O)C(=O)N[C@@H](CC)C(=O)O)SC)C4=CC=CC=C4)CCCC
|
| InChi Key |
XULSCZPZVQIMFM-IPZQJPLYSA-N
|
| InChi Code |
InChI=1S/C37H48N4O8S2/c1-5-8-19-37(20-9-6-2)24-41(26-13-11-10-12-14-26)29-21-31(50-4)30(22-32(29)51(47,48)40-37)49-23-33(43)39-34(25-15-17-27(42)18-16-25)35(44)38-28(7-3)36(45)46/h10-18,21-22,28,34,40,42H,5-9,19-20,23-24H2,1-4H3,(H,38,44)(H,39,43)(H,45,46)/t28-,34+/m0/s1
|
| 化学名 |
(S)-2-((R)-2-(2-((3,3-dibutyl-7-(methylthio)-1,1-dioxido-5-phenyl-2,3,4,5-tetrahydrobenzo[f][1,2,5]thiadiazepin-8-yl)oxy)acetamido)-2-(4-hydroxyphenyl)acetamido)butanoic acid
|
| 别名 |
AZD8294; A4250; AR-H064974; Odevixibat; 501692-44-0; A4250; AZD8294; AR-H064974; Odevixibat [USAN]; 2W150K0UUC;AZD-8294; A-4250; AR-H-064974; Bylvay
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~166.67 mg/mL (~224.95 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 4.17 mg/mL (5.63 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 41.7 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 4.17 mg/mL (5.63 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 41.7mg/mL澄清DMSO储备液加入900μL玉米油中,混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3497 mL | 6.7483 mL | 13.4966 mL | |
| 5 mM | 0.2699 mL | 1.3497 mL | 2.6993 mL | |
| 10 mM | 0.1350 mL | 0.6748 mL | 1.3497 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05426733 | ENROLLING BY INVITATION | Drug: Odevixibat | Biliary Atresia | Albireo, an Ipsen Company | 2022-07-05 | Phase 3 |
| NCT05035030 | RECRUITING | Drug: Odevixibat | Alagille Syndrome | Albireo, an Ipsen Company | 2021-09-03 | Phase 3 |
| NCT04483531 | APPROVED FOR MARKETING | Drug: Odevixibat | Progressive Familial Intrahepatic Cholestasis | Albireo | ||
| NCT04674761 | COMPLETEDWITH RESULTS | Drug: Odevixibat Drug: Placebo |
Alagille Syndrome | Albireo | 2021-03-19 | Phase 3 |
| NCT04336722 | ACTIVE, NOT RECRUITING | Drug: Odevixibat Drug: Placebo |
Biliary Atresia | Albireo, an Ipsen Company | 2020-07-08 | Phase 3 |