| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|

| 体外研究 (In Vitro) |
体外活性:Olmesartan Medoxomil 显着降低肝脏羟脯氨酸含量、胶原蛋白 α1(I) 和 α-平滑肌肌动蛋白 (α-SMA) 的 mRNA 表达以及转化生长因子-β1 (TGF-β1) 的血浆水平。 Olmesartan Medoxomil 是一种含有酯部分的前药,口服后迅速裂解释放活性形式奥美沙坦 (RNH-6270)。奥美沙坦是一种高效、竞争性和选择性的 All AT1 受体拮抗剂,对 AT2 和 AT4 受体几乎没有拮抗活性。激酶测定:Olmesartan medoxomil 是一种有效的选择性血管紧张素 AT1 受体抑制剂,IC50 为 66.2 μM。
|
||
|---|---|---|---|
| 体内研究 (In Vivo) |
奥美沙坦对清醒大鼠中 All 诱导的升压反应产生快速且持久的抑制。 Oralolmesartan medoxomil 也抑制全升压反应,但与静脉内给药相比,起效较慢。 Olmesartan Medoxomil 在多种大鼠和狗模型中表现出剂量依赖性抗高血压作用,与正常或低肾素类型相比,在高血浆肾素模型中观察到最显着的效果。奥美沙坦酯除了具有抗高血压作用外,还在各种类型肾病和心力衰竭的动物模型中表现出有益作用,并且在高脂血症动物中具有抗动脉粥样硬化作用。 Olmesartan Medoxomil 剂量依赖性地改善大鼠结肠组织病理学和生化损伤,其效果与标准柳氮磺吡啶相当甚至更好。奥美沙坦酯不仅在超声心动图观察中显着降低了缺氧性肺心病的诱导,而且在分子研究中也显着降低了慢性缺氧大鼠的脑利钠肽(BNP)、TGF-β和内皮素基因表达。
|
||
| 酶活实验 |
Olmesartan medoxomil 的 IC50 为 66.2 μM,是一种强效、特异性的血管紧张素 AT1 受体抑制剂。
|
||
| 细胞实验 |
细胞系:人宫颈癌细胞系 (HeLa)
浓度:0.7- 5 mM 孵育时间:24、48 和 72 小时 结果:48 和 48 小时对 HeLa 细胞系的 IC50 分别为 4.685 和 1.651 mM分别为72小时 |
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
When taken orally, the prodrug olmesartan medoxomil is rapidly absorbed in the gastrointestinal tract and metabolized to olmesartan. The esterification with medoxomil was created with the intention of increasing olmesartan bioavailability from 4.5% to 28.6%. Oral administration of 10-160 mg of olmesartan has been shown to reach peak plasma concentration of 0.22-2.1 mg/L after 1-3 hours with an AUC of 1.6-19.9mgh/L. The pharmacokinetic profile of olmesartan has been observed to be nearly linear and dose-dependent under the therapeutic range. The steady-state level of olmesartan is achieved after once a day dosing during 3 to 5 days. The main elimination route of olmesartan is in the unchanged form through the feces. From the systemically bioavailable dose, about 10-16% is eliminated in the urine. 17 L Total plasma clearance is 1.3 L/h and the renal clearance is 0.6 L/h. In rats, olmesartan crossed the blood-brain barrier poorly, if at all. Olmesartan passed across the placental barrier in rats and was distributed to the fetus. The volume of distribution of olmesartan is approximately 17 L. Olmesartan is highly bound to plasma proteins (99%) and does not penetrate red blood cells. The protein binding is constant at plasma olmesartan concentrations well above the range achieved with recommended doses. Olmesartan medoxomil is rapidly and completely bioactivated by ester hydrolysis to olmesartan during absorption from the gastrointestinal tract. Benicar tablets and the suspension formulation prepared from Benicar tablets are bioequivalent. The absolute bioavailability of olmesartan is approximately 26%. After oral administration, the peak plasma concentration (Cmax ) of olmesartan is reached after 1 to 2 hours. Food does not affect the bioavailability of olmesartan. Total plasma clearance of olmesartan is 1.3 L/hr, with a renal clearance of 0.6 L/hr. Approximately 35% to 50% of the absorbed dose is recovered in urine while the remainder is eliminated in feces via the bile. ... Olmesartan shows linear pharmacokinetics following single oral doses of up to 320 mg and multiple oral doses of up to 80 mg. Steady-state levels of olmesartan are achieved within 3 to 5 days and no accumulation in plasma occurs with once-daily dosing. Olmesartan is distributed into milk in rats; it is not known whether the drug is distributed into milk in humans. Metabolism / Metabolites Olmesartan medoxomil is rapidly and completely bioactivated by ester hydrolysis to olmesartan during absorption from the gastrointestinal tract. This rapid first-pass metabolism was confirmed by the lack of measurable amounts of olmesartan medoxomil in plasma or excreta. This first-pass metabolism is not driven by cytochrome enzymes and hence it is not expected to interact with other drugs via this mechanism. The pharmacologically active moiety does not appear to undergo further metabolism. Following the rapid and complete conversion of olmesartan medoxomil to olmesartan during absorption, there is virtually no further metabolism of olmesartan. Biological Half-Life The mean plasma olmesartan half-life is reported to be from 10-15 hours after multiple oral administration. Olmesartan appears to be eliminated in a biphasic manner with a terminal elimination half-life of approximately 13 hours. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Olmesartan is a white to light yellowish-powder or crystalline powder that is formulated into oral tablets. Olmesartan is an angiotensin II type 1 (AT1) receptor antagonist. It is used alone or in combination with other classes of antihypertensive agents (e.g., thiazide diuretics) in the management of hypertension. HUMAN EXPOSURE AND TOXICITY: Limited data are available related to drug overdose in humans. The most likely manifestations of olmesartan overdose include hypotension and tachycardia; bradycardia could be encountered if parasympathetic (vagal) stimulation occurs. While olmesartan can be used in hypertensive children, it is not approved for use in children less one 1 year of age. Drugs that act directly on the renin-angiotensin system (RAS) can have effects on the development of immature kidneys. The use of olmesartan in pregnancy is also contraindicated. While use during the first trimester does not suggest a risk of major anomalies, use during the second and third trimester may cause teratogenicity and severe fetal and neonatal toxicity. Fetal toxic effects may include anuria, oligohydramnios, fetal hypocalvaria, intrauterine growth restriction, premature birth, and patent ductus arteriosus. Anuria-associated anhydramnios/oligohydramnios may produce fetal limb contractures, craniofacial deformation, and pulmonary hypoplasia. Severe anuria and hypotension, resistant to both pressor agents and volume expansion, may occur in the newborn following in utero exposure to olmesartan. ANIMAL STUDIES: Olmesartan was not carcinogenic when administered by dietary administration to rats for up to 2 years. Also, the fertility of male and female rats was unaffected by administration of olmesartan. No teratogenic effects were observed when drug was administered to pregnant rats at oral doses up to 1000 mg/kg/day or pregnant rabbits at oral doses up to 1 mg/kg/day. However, significant decreases in pup birth weight and weight gain were observed in rats. In addition, delays in developmental milestones (delayed separation of ear auricula, eruption of lower incisors, appearance of abdominal hair, descent of testes, and separation of eyelids) and dose-dependent increases in the incidence of dilation of the renal pelvis were observed in rats. Olmesartan tested negative in the in vitro Syrian hamster embryo cell transformation assay and showed no evidence of genetic toxicity in the Ames (bacterial mutagenicity) test. However, the drug was shown to induce chromosomal aberrations in cultured cells in vitro (Chinese hamster lung) and tested positive for thymidine kinase mutations in the in vitro mouse lymphoma assay. Olmesartan tested negative in vivo for mutations in the MutaMouse intestine and kidney and for clastogenicity in mouse bone marrow (micronucleus test) at oral doses of up to 2000 mg/kg. Hepatotoxicity Olmesartan has been associated with a low rate of serum aminotransferase elevations ( Likelihood score: D (Possible rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation With the exception of one adverse reaction in a breastfed infant, no information is available on the use of olmesartan during breastfeeding. An alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants A 6-day-old healthy full-term newborn was exposed to olmesartan in breastmilk. The maternal dose of olmesartan and extent of nursing were not reported. Weight gain was normal during the first two weeks of life, but at the pediatric checkup on the 17th day postpartum, an abrupt decrease in body weight was recorded. He was hospitalized on the 21st day, and mixed feeding with milk and formula was started. Biochemical examinations showed aspartate-aminotransferase (AST) 250 mg/dL, and regular urinalysis. Virologic and metabolic causes of elevated transaminase were ruled out. The baby started to regain weight and his AST gradually normalized to 50 mg/dL by the 24th day. Olmesartan intake was stopped and the child was discharged on the 24th day of life. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Olmesartan is highly bound to plasma proteins. 99% of the administered dose is found in a bound state with no penetration in red blood cells. Interactions Do not co-administer aliskiren with Benicar in patients with diabetes. Avoid use of aliskiren with Benicar in patients with renal impairment (GFR <60 mL/min). Dual Blockade of the renin-angiotensin system (RAS)with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Most patients receiving the combination of two RAS inhibitors do not obtain any additional benefit compared to monotherapy. In general, avoid combined use of RAS inhibitors. Closely monitor blood pressure, renal function and electrolytes in patients on Benicar and other agents that affect the RAS. Increases in serum lithium concentrations and lithium toxicity have been reported during concomitant administration of lithium with angiotensin II receptor antagonists, including Benicar. Monitor serum lithium levels during concomitant use. Potential pharmacologic interaction (attenuated hypotensive effects) when angiotensin II receptor antagonists are used concomitantly with nonsteroidal anti-inflammatory agents (NSAIAs), including selective cyclooxygenase-2 (COX-2) inhibitors. Possible deterioration of renal function, including possible acute renal failure, in geriatric, volume-depleted (including those receiving concomitant diuretic therapy), or renally impaired patients; renal function should be monitored periodically in patients receiving concomitant therapy with olmesartan and an NSAIA, including selective COX-2 inhibitors. For more Interactions (Complete) data for Olmesartan (10 total), please visit the HSDB record page. |
||
| 参考文献 |
|
||
| 其他信息 |
Therapeutic Uses
Angiotensin II Type 1 Receptor Blockers; Antihypertensive Agents Benicar is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including the class to which this drug principally belongs. There are no controlled trials demonstrating risk reduction with Benicar. Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program's Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC). Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly. Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment a lower blood pressure goal. Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy. It may be used alone or in combination with other antihypertensive agents. /Included in US product label/ Both angiotensin II receptor antagonists /including olmesartan/ and ACE inhibitors have been shown to slow the rate of progression of renal disease in hypertensive patients with diabetes mellitus and microalbuminuria or overt nephropathy, and use of a drug from either class is recommended in such patients. /NOT included in US product label/ Angiotensin II receptor antagonists /inlcuding olmesartan/ have been used with equivocal results in the management of congestive heart failure. While angiotensin II receptor antagonists appear to share the hemodynamic effects of ACE inhibitors, some clinicians state that in the absence of data documenting comparable long-term cardiovascular and/or renal benefits, angiotensin II receptor antagonists should be reserved principally for patients in whom ACE inhibitors are indicated but who are unable to tolerate the drugs (e.g., because of cough). /NOT included in US product label/ The antihypertensive effects of Benicar in the pediatric population were evaluated in a randomized, double-blind study involving 302 hypertensive patients aged 6 to 16 years. The study population consisted of an all black cohort of 112 patients and a mixed racial cohort of 190 patients, including 38 blacks. The etiology of the hypertension was predominantly essential hypertension (87% of the black cohort and 67% of the mixed cohort). Patients who weighed 20 to <35 kg were randomized to 2.5 or 20 mg of Benicar once daily and patients who weighed > or = 35 kg were randomized to 5 or 40 mg of Benicar once daily. At the end of 3 weeks, patients were re-randomized to continuing Benicar or to taking placebo for up to 2 weeks. During the initial dose-response phase, Benicar significantly reduced both systolic and diastolic blood pressure in a weight-adjusted dose-dependent manner. Overall, the two dose levels of Benicar (low and high) significantly reduced systolic blood pressure by 6.6 and 11.9 mm Hg from the baseline, respectively. These reductions in systolic blood pressure included both drug and placebo effect. During the randomized withdrawal to placebo phase, mean systolic blood pressure at trough was 3.2 mm Hg lower and mean diastolic blood pressure at trough was 2.8 mm Hg lower in patients continuing Benicar than in patients withdrawn to placebo. These differences were statistically different. As observed in adult populations, the blood pressure reductions were smaller in black patients. In the same study, 59 patients aged 1 to 5 years who weighed > or = 5 kg received 0.3 mg/kg of Benicar once daily for three weeks in an open label phase and then were randomized to receiving Benicar or placebo in a double-blind phase. At the end of the second week of withdrawal, the mean systolic/diastolic blood pressure at trough was 3/3 mm Hg lower in the group randomized to Benicar; this difference in blood pressure was not statistically significant (95% C.I. -2 to 7/-1 to 7). Drug Warnings /BOXED WARNING/ WARNING: FETAL TOXICITY. When pregnancy is detected, discontinue Benicar as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Benicar as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus. In the unusual case that there is no appropriate alternative to therapy with drugs affecting the reninangiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue Benicar, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Neonates with a history of in utero exposure to Benicar: If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function. FDA Pregnancy Risk Category: D /POSITIVE EVIDENCE OF RISK. Studies in humans, or investigational or post-marketing data, have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective./ For more Drug Warnings (Complete) data for Olmesartan (19 total), please visit the HSDB record page. Pharmacodynamics Overall, olmesartan's physiologic effects lead to reduced blood pressure, lower aldosterone levels, reduced cardiac activity, and increased excretion of sodium. **Hypotension in Volume- or Salt-Depleted Patients** In patients with an activated renin-angiotensin aldosterone system, such as volume-and/or salt-depleted patients (e.g., those being treated with high doses of diuretics), symptomatic hypotension may be anticipated after initiation of treatment with olmesartan. Initiate treatment under close medical supervision. If hypotension does occur, place the patient in the supine position and, if necessary, give an intravenous infusion of normal saline. A transient hypotensive response is not a contraindication to further treatment, which usually can be continued without difficulty once the blood pressure has stabilized. Valvular Stenosis: there is concern on theoretical grounds that patients with aortic stenosis might be at a particular risk of decreased coronary perfusion, because they do not develop as much afterload reduction. **Impaired Renal Function** As a consequence of inhibiting the renin-angiotensin-aldosterone system, changes in renal function may be anticipated in susceptible individuals treated with olmesartan. In patients whose renal function may depend upon the activity of the renin-angiotensin- aldosterone system (e.g., patients with severe congestive heart failure), treatment with angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor antagonists has been associated with oliguria and/or progressive azotemia and rarely with acute renal failure and/or death. Similar results may be anticipated in patients treated with olmesartan. In studies of ACE inhibitors in patients with unilateral or bilateral renal artery stenosis, increases in serum creatinine or blood urea nitrogen (BUN) have been reported. There has been no long-term use of olmesartan medoxomil in patients with unilateral or bilateral renal artery stenosis, but similar results may be expected. **Sprue-like Enteropathy** Severe, chronic diarrhea with substantial weight loss has been reported in patients taking olmesartan months to years after drug initiation. Intestinal biopsies of patients often demonstrated villous atrophy. If a patient develops these symptoms during treatment with olmesartan, exclude other etiologies. Consider discontinuation of olmesartan medoxomil in cases where no other etiology is identified. **Electrolyte Imbalances** Olmesartan medoxomil contains olmesartan, a drug that inhibits the renin-angiotensin system (RAS). Drugs that inhibit the RAS can cause hyperkalemia. Monitor serum electrolytes periodically. |
| 分子式 |
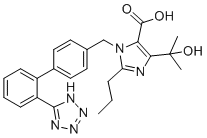
C24H26N6O3
|
|
|---|---|---|
| 分子量 |
446.50
|
|
| 精确质量 |
446.206
|
|
| 元素分析 |
C, 64.56; H, 5.87; N, 18.82; O, 10.75
|
|
| CAS号 |
144689-24-7
|
|
| 相关CAS号 |
Olmesartan-d4; 1420880-41-6; Olmesartan-d6; 1185144-74-4; Olmesartan medoxomil; 144689-63-4
|
|
| PubChem CID |
158781
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
738.3±70.0 °C at 760 mmHg
|
|
| 熔点 |
186-188ºC
|
|
| 闪点 |
400.3±35.7 °C
|
|
| 蒸汽压 |
0.0±2.6 mmHg at 25°C
|
|
| 折射率 |
1.671
|
|
| LogP |
3.72
|
|
| tPSA |
129.81
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
8
|
|
| 重原子数目 |
33
|
|
| 分子复杂度/Complexity |
656
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O([H])C(C([H])([H])[H])(C([H])([H])[H])C1=C(C(=O)O[H])N(C([H])([H])C2C([H])=C([H])C(C3=C([H])C([H])=C([H])C([H])=C3C3N=NN([H])N=3)=C([H])C=2[H])C(C([H])([H])C([H])([H])C([H])([H])[H])=N1
|
|
| InChi Key |
VTRAEEWXHOVJFV-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C24H26N6O3/c1-4-7-19-25-21(24(2,3)33)20(23(31)32)30(19)14-15-10-12-16(13-11-15)17-8-5-6-9-18(17)22-26-28-29-27-22/h5-6,8-13,33H,4,7,14H2,1-3H3,(H,31,32)(H,26,27,28,29)
|
|
| 化学名 |
5-(2-hydroxypropan-2-yl)-2-propyl-3-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]imidazole-4-carboxylic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.60 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.60 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.60 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2396 mL | 11.1982 mL | 22.3964 mL | |
| 5 mM | 0.4479 mL | 2.2396 mL | 4.4793 mL | |
| 10 mM | 0.2240 mL | 1.1198 mL | 2.2396 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Host Response Mediators in Coronavirus (COVID-19) Infection - Is There a Protective Effect of Losartan and Other ARBs on Outcomes of Coronavirus Infection?
CTID: NCT04606563
Phase: Phase 3 Status: Terminated
Date: 2023-02-16
|
|
|