| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
Nrf2; NF-κB
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:Omaveloxolone(RTA-408)有效增加 Nrf2 靶基因的表达,并逆转 RAW 264.7 细胞中 IFNγ 介导的 Gclc 表达抑制。在一组八种人类肿瘤细胞系中,RTA 408 抑制生长,平均 GI50 值为 260 nM,并诱导细胞凋亡。 RTA 408 还抑制肿瘤细胞中的 NF-κB 并激活 JNK。细胞测定:对于生长抑制测定,将细胞以每孔 3 x 103 个细胞的密度铺在重复的 96 孔培养皿中。第二天,一个板用 RTA 408 处理,另一个板立即进行磺酰罗丹明 B (SRB) 测定(时间 0)。处理开始 72 小时后,对 RTA 408 处理的板中的细胞进行 SRB 测定。使用以下公式计算相对于载体处理细胞的生长百分比:[(Ti-Tz)/(C-Tz)] x 100,其中 (Tz) 是时间零时的吸光度值,(C) 是载体处理孔的吸光度值72小时后,(Ti)是用药物处理的孔的吸光度值。在 GraphPad Prism 中绘制剂量反应曲线并计算 GI50 值。
在低浓度(≤25 nM)下,RTA 408激活Nrf2,抑制干扰素-γ刺激的RAW 264.7巨噬细胞中的一氧化氮和促炎细胞因子水平。在较高浓度下,RTA 408抑制了肿瘤细胞生长(GI50=260±74 nM),并增加了肿瘤细胞系中的胱天蛋白酶活性,但在正常原代人类细胞中没有。与AIMs对IKKβ的直接作用一致,RTA 408在抑制细胞生长和诱导凋亡的相同浓度下抑制NF-κB信号传导并降低细胞周期蛋白D1水平。RTA 408还增加了CDKN1A(p21)水平和JNK磷酸化。RTA 408的体外活性曲线类似于甲基巴多索龙,在证明靶向参与的剂量下,患者对其耐受良好。总之,这些数据支持用于癌症治疗的RTA 408的临床评价。[1] |
| 体内研究 (In Vivo) |
在患有放射性皮炎的小鼠中,1.0%Omaveloxolone(RTA-408) 显着减少表皮和胶原增厚,防止真皮坏死并完全缓解皮肤溃疡。在大鼠皮肤中,RTA 408 激活 Nrf2 并诱导细胞保护基因。 RTA 408 还可以减轻小鼠的造血急性辐射综合征。
本研究旨在确定新型齐墩烷三萜类化合物Omaveloxolone(RTA-408)是否以及如何对雄性小鼠的肾缺血再灌注损伤(IRI)提供保护。与赋形剂治疗的小鼠相比,用Omaveloxolone(RTA-408)治疗的小鼠在单侧缺血后进行对侧肾切除术,肾功能和组织学结果得到改善,凋亡、ROS产生和氧化损伤标志物减少。此外,我们发现RTA-408可以通过增加Nrf2核转位来增强总抗氧化能力,从而增加Nrf2下游GSH相关抗氧化基因的表达和活性。体外研究表明,GSH合成酶GCLc可能是RTA-408的重要靶点。此外,用RTA-408治疗的Nrf2缺陷小鼠在肾功能、组织学、ROS产生和GSH相关基因表达方面没有显著改善。因此,通过上调Nrf2及其下游抗氧化基因,RTA-408为肾脏IRI的预防和治疗提供了一种新的潜在方法[2]。 |
| 酶活实验 |
NF-κB信号传导[1]
对于NF-κB-萤光素酶报告基因检测,将HeLa NF-κB-Luc(每孔1.9 x 104个细胞)和A549/NF-κB-Luc(每孔1.6 x 104个电池)细胞接种在底部透明的96孔黑色平板中。24小时后,细胞用DMSO或几种浓度的Omaveloxolone(RTA-408)预处理1小时,然后用10ng/ml的人TNFα再处理5小时。根据制造商的说明,使用One Glo萤光素酶测定法测量萤火虫萤光素酶活性。对于IκBα蛋白质印迹,将HeLa细胞以每孔1 x 105个细胞的密度接种在24孔培养皿中。第二天,细胞用DMSO或几种浓度的Omaveloxolone(RTA-408)或甲基巴多索龙预处理6小时。然后用20ng/ml的人TNFα处理细胞5分钟。细胞立即在含有2%BME的Tricine样品缓冲液中裂解,并如上所述进行蛋白质印迹处理。 抗氧化能力测定[2] 根据制造商的说明,使用每个检测试剂盒估算肾皮质匀浆上清液中丙二醛(MDA)、羰基化蛋白、总抗氧化能力(T-AOC)、总谷胱甘肽(T-GSH)和谷胱甘肽与谷胱甘肽二硫化物(GSH/GSSG)的比率。 酶活性测定[2] 还使用市售的检测试剂盒测量了肾皮质匀浆上清液中GSH相关酶的活性。 |
| 细胞实验 |
将细胞以每孔 3 x 103 个铺板于重复的 96 孔培养皿中,用于生长抑制测定。第二天,一个板接受 Omaveloxolone(RTA-408)的应用,而另一个板立即进行磺基罗丹明 B (SRB) 测定(时间 0)。用 RTA 408 处理 72 小时后,板上的细胞准备用于 SRB 测定。公式 [(Ti-Tz)/(C-Tz)] x 100 用于计算与用载体处理的细胞相比的生长百分比。其中 (C) 是 72 小时后媒介物处理孔的吸光度值,(Ti) 是用药物处理的孔的吸光度值,(Tz) 是零时间时的吸光度值。在 GraphPad Prism 中,绘制剂量反应曲线,并计算 GI50 值。
|
| 动物实验 |
Mice: Wild-type C57Bl/6 CD45.2 mice are used, which are 6–8 weeks old, for radiation survival tests. In transplantation experiments, recipients include congenic wild-type C57Bl/6 CD45.1 host mice and C57Bl/6 CD45.1/CD45.2 hybrid host mice. For vehicle control, omeveloxolone stock solutions (DMSO) are made within an hour of injection. At 24, 48, and 72 hours after irradiation, intraperitoneal administration of DMSO or omaveloxolone (17.5 mg/kg) is performed. A 250-kVp X-ray machine with a 50 cm source-to-skin distance and a 2 mm copper filter is used to administer whole-body irradiation (7-8 Gy). About 1.4 Gy/min is the dose rate.
Mouse Model of Ischemia-Reperfusion Injury[2] 24 h before surgery, mice were intraperitoneally administered with RTA-408 (100 μg/kg body weight) or 0.1% dimethyl sulfoxide (DMSO) in PBS as the vehicle. The rationale for RTA-408 dosage was based on the renal function preservation and Nrf2 mRNA activation. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Between 50 mg (0.33 times the recommended dosage) and 150 mg, the AUC of omaveloxolone increased in a dose-dependent and dose-proportional manner. However, at the same dose range, the Cmax increased in a less than dose-proportional manner in healthy fasted subjects. The median time to achieve peak plasma concentration was 7 to 14 hours. Compared to fasted conditions, the AUC0-inf and Cmax of omaveloxolone were 350% and 15% higher with a high-fat meal (800 to 1000 calories, with 150, 250, and 500 to 600 calories coming from protein, carbohydrate, and fat, respectively). Omaveloxolone is mainly excreted in feces. In healthy subjects given a single oral dose of radiolabeled omaveloxolone (150 mg), about 92% and 0.1% of the dose were recovered in feces and urine, respectively. Approximately 91% of the omaveloxolone found in feces was recovered within 96 hours after administration. Omaveloxolone has an apparent volume of distribution of 7361 L. Omaveloxolone has an apparent plasma clearance of 109 L/hr. Metabolism / Metabolites Omaveloxolone is mainly metabolized by CYP3A, although CYP2C8 and CYP2J2 play also a minor role. Biological Half-Life Omaveloxolone has a terminal half-life of 57 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the clinical use of omaveloxolone during breastfeeding. Because it is 97% protein bound, exposure of the breastfed infant is likely to be low. Until more data become available, omaveloxolone should be used with caution during breastfeeding, especially while nursing a newborn or preterm infant ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding The protein binding of omaveloxolone is 97%. |
| 参考文献 |
|
| 其他信息 |
Omaveloxolone is a semi-synthetic triterpenoid drug. It is an Nrf2 activator that is approved for the treatment of Friedreich's ataxia in adults and adolescents aged 16 years and older. It has a role as an antioxidant, an anti-inflammatory agent, a cardioprotective agent and an antineoplastic agent. It is a pentacyclic triterpenoid, a secondary carboxamide, a nitrile, an organofluorine compound and a cyclic terpene ketone. It derives from a hydride of an oleanane.
Omaveloxolone (RTA-408) is a semisynthetic oleanane triterpenoid with antioxidant and anti-inflammatory properties. Omaveloxolone acts as an activator of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), a transcription factor that mitigates oxidative stress. In patients with Friedreich's ataxia, a genetic disease involving mitochondrial dysfunction, the Nrf2 pathway is impaired, and Nrf2 activity is lower. Therefore, the use of Nrf2 activators such as omaveloxolone represents a therapeutic advantage in this group of patients. In February 2023, omaveloxolone was approved by the FDA for the treatment of Friedreich's ataxia in adults and adolescents aged 16 years and older. The use of omaveloxolone for the treatment of conditions involving mitochondrial dysfunction and oxidative stress has also been evaluated. The mechanism of action of omaveloxolone is as a Cytochrome P450 3A4 Inducer, and Cytochrome P450 2C8 Inducer. Omaveloxolone is a member of the synthetic oleanane triterpenoid class of compounds and an activator of nuclear factor erythroid 2 [NF-E2]-related factor 2 (Nrf2, Nfe2l2), with potential chemopreventive activity. Upon administration, omaveloxolone activates the cytoprotective transcription factor Nrf2. In turn, Nrf2 translocates to the nucleus, dimerizes with a small Maf protein (sMaf), and binds to the antioxidant response element (ARE). This induces the expression of a number of cytoprotective genes, including NAD(P)H quinone oxidoreductase 1 (NQO1), sulfiredoxin 1 (Srxn1), heme oxygenase-1 (HO1, HMOX1), superoxide dismutase 1 (SOD1), gamma-glutamylcysteine synthetase (gamma-GCS), thioredoxin reductase-1 (TXNRD1), glutathione S-transferase (GST), glutamate-cysteine ligase catalytic subunit (Gclc) and glutamate-cysteine ligase regulatory subunit (Gclm), and increases the synthesis of the antioxidant glutathione (GSH). Nrf2, a leucine zipper transcription factor, plays a key role in the maintenance of redox balance and cytoprotection against oxidative stress. Drug Indication Omaveloxolone is indicated for the treatment of Friedreich's ataxia in adults and adolescents aged 16 years and older. Treatment of Friedreich's ataxia Mechanism of Action The mechanism of action of omaveloxolone has not been fully elucidated; however, its therapeutic effect in patients with Friedreich's ataxia may be linked to its ability to activate the Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway. Nrf2 is a transcription factor that mitigates oxidative stress. In normal conditions, Nrf2 levels are regulated by Kelch-like ECH-associated protein 1 (KEAP1), which binds to Nrf2, prevents Nrf2 translocation to the nucleus, and degrades it by ubiquitination. In the presence of oxidative stress, the ubiquitination system is disrupted. Nrf2 accumulates and translocates to the nucleus, where it promotes the expression of genes against oxidative stress. In patients with Friedreich's ataxia, the Nrf2 signaling pathway is dysfunctional. In Friedreich's ataxia models, Nrf2 has a lower activity; therefore, Nrf2 activators such as omaveloxolone represent a therapeutic opportunity. A study has shown that omaveloxolone binds to KEAP1, inhibiting its interaction with Nrf2 and promoting the translocation of Nrf2 to the nucleus. Aside from activating Nrf2, omaveloxolone also inhibits the NF-κB signalling pathway, promoting antioxidative, anti-inflammatory, and antiapoptotic mechanisms. Pharmacodynamics The MOXIe study showed that after 4 weeks of administration, the use of omaveloxolone led to robust dose‐dependent changes at 80 ‐300 mg/day.. The effect of omaveloxolone on QTc interval has yet to be defined. The use of omaveloxolone can lead to an elevation in hepatic transaminases (both aspartate transaminase and alanine transaminase) and an increase in B-type natriuretic peptide (BNP), a marker of cardiac function. It can also cause changes in cholesterol. |
| 分子式 |
C33H44F2N2O3
|
|
|---|---|---|
| 分子量 |
554.71
|
|
| 精确质量 |
554.331
|
|
| 元素分析 |
C, 71.45; H, 8.00; F, 6.85; N, 5.05; O, 8.65
|
|
| CAS号 |
1474034-05-3
|
|
| 相关CAS号 |
|
|
| PubChem CID |
71811910
|
|
| 外观&性状 |
White solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
662.0±55.0 °C at 760 mmHg
|
|
| 闪点 |
354.2±31.5 °C
|
|
| 蒸汽压 |
0.0±2.0 mmHg at 25°C
|
|
| 折射率 |
1.549
|
|
| LogP |
5.64
|
|
| tPSA |
87
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
40
|
|
| 分子复杂度/Complexity |
1320
|
|
| 定义原子立体中心数目 |
7
|
|
| SMILES |
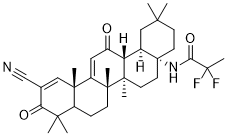
O=C1C(C#N)=C[C@@]2(C)[C@](CC[C@]([C@@]3(C)[C@@]4([H])[C@@]5([H])[C@@](CCC(C)(C)C5)(NC(C(F)(F)C)=O)CC3)(C)C2=CC4=O)([H])C1(C)C
|
|
| InChi Key |
RJCWBNBKOKFWNY-IDPLTSGASA-N
|
|
| InChi Code |
InChI=1S/C33H44F2N2O3/c1-27(2)11-13-33(37-26(40)32(8,34)35)14-12-31(7)24(20(33)17-27)21(38)15-23-29(5)16-19(18-36)25(39)28(3,4)22(29)9-10-30(23,31)6/h15-16,20,22,24H,9-14,17H2,1-8H3,(H,37,40)/t20-,22-,24-,29-,30+,31+,33-/m0/s1
|
|
| 化学名 |
N-[(4aS,6aR,6bS,8aR,12aS,14aR,14bS)-11-cyano-2,2,6a,6b,9,9,12a-heptamethyl-10,14-dioxo-1,3,4,5,6,7,8,8a,14a,14b-decahydropicen-4a-yl]-2,2-difluoropropanamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.51 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 配方 2 中的溶解度: 10% DMSO +90%Corn oil: 30mg/mL 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8027 mL | 9.0137 mL | 18.0274 mL | |
| 5 mM | 0.3605 mL | 1.8027 mL | 3.6055 mL | |
| 10 mM | 0.1803 mL | 0.9014 mL | 1.8027 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02255435 | Active Recruiting |
Drug: Placebo Drug: Omaveloxolone Capsules, 20 mg |
Friedreich Ataxia | Reata Pharmaceuticals, Inc. | January 2015 | Phase 2 |
| NCT06054893 | Not yet recruiting | Drug: Omaveloxolone | Friedreich Ataxia | Reata Pharmaceuticals, Inc. | November 2023 | Phase 1 |
| NCT03902002 | Completed | Drug: Omaveloxolone 50 mg capsules |
Hepatic Impairment | Reata Pharmaceuticals, Inc. | July 19, 2019 | Phase 1 |
| NCT03664453 | Completed | Drug: omaveloxolone | Healthy | Reata Pharmaceuticals, Inc. | July 19, 2019 | Phase 1 |
| NCT05909644 | Completed | Drug: omaveloxolone Drug: Efavirenz |
Healthy Adult Subjects | Reata Pharmaceuticals, Inc. | July 5, 2023 | Phase 1 |
|
|
|
|
|
|
|