| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| 1g | |||
| Other Sizes |
| 靶点 |
Neuroprotective agent
|
|---|---|
| 体外研究 (In Vitro) |
在 PC12 细胞中,P7C3-A20(10-100 μM;8 小时)处理可减轻氧还原增强 (OGD) 产生的细胞毒性 [1]。
|
| 体内研究 (In Vivo) |
在 HI 范例中,P7C3-A20(5-10 mg/kg;腹膜内;每天;7 天;Sprague-Dawley 大鼠)减少梗塞数量,逆转视网膜和海马细胞的损失,并增强运动功能。然而,P7C3–A20 无法通过开启 PI3K/AKT/GSK3β 信号传导来阻止 HI 诱导的神经元损伤 [1]。
|
| 酶活实验 |
新生儿缺氧缺血性脑病(HIE)可导致严重的长期残疾,包括脑瘫和脑损伤。小分子P7C3-A20已被证明在各种疾病中具有神经保护作用,如缺血性中风和神经退行性疾病。然而,目前尚不清楚P7C3-A20是否具有治疗HIE的潜力,P7C3-A2与神经元凋亡之间的关系尚不清楚。为了解决这些问题,本研究调查了P7C3-A20是否在体外使用PC12细胞氧葡萄糖剥夺(OGD)模型和在出生后第7天和第14天接受HI的大鼠体内减少HI损伤,以及潜在的机制。我们发现,用P7C3-A20(40-100µM)治疗减轻了OGD诱导的PC12细胞凋亡。在HI模型大鼠中,用5或10mg/kg P7C3-A20治疗可减少梗死体积;逆转了皮质和海马区的细胞损失,改善了运动功能,而不会引起神经毒性。用磷脂酰肌醇3-激酶(PI3K)抑制剂LY294002治疗可消除神经保护作用。这些结果表明,P7C3-A20通过激活PI3K/蛋白激酶B/糖原合酶激酶3β信号传导发挥神经保护作用,并可能用于预防HIE后新生儿的脑损伤[1]。
|
| 细胞实验 |
细胞活力测定[1]
细胞类型: PC12 细胞 测试浓度: 10 μM、20 μM、40 μM、60 μM、80 μM、100 μM 孵育持续时间:8 小时)处理可以减弱 OGD 诱导的 PC12 细胞不育性[1]。 实验结果:减轻氧糖剥夺 (OGD) 诱导的 PC12 细胞细胞毒性。 细胞凋亡分析 [1] 细胞类型: PC12 细胞 测试浓度: 40 μM、60 μM、80 μM、100 μM 孵育持续时间:8小时 实验结果:减少氧糖剥夺(OGD)诱导的PC12细胞凋亡。 |
| 动物实验 |
Animal/Disease Models: SD (SD (Sprague-Dawley)) rat (200-250 g) induced hypoxic-ischemic (HI) injury [1]
Doses: 5 mg/kg, 10 mg/kg Route of Administration: intraperitoneal (ip) injection; daily; continued for 7 Day Experimental Results: Reduction in infarct volume; reversal of cell loss in cortex and hippocampus and improvement in motor function without causing neurotoxicity. |
| 参考文献 |
|
| 其他信息 |
Traumatic brain injury (TBI) is characterized by histopathological damage and long-term sensorimotor and cognitive dysfunction. Recent studies have reported the discovery of the P7C3 class of aminopropyl carbazole agents with potent neuroprotective properties for both newborn neural precursor cells in the adult hippocampus and mature neurons in other regions of the central nervous system. This study tested, for the first time, whether the highly active P7C3-A20 compound would be neuroprotective, promote hippocampal neurogenesis, and improve functional outcomes after experimental TBI. Sprague-Dawley rats subjected to moderate fluid percussion brain injury were evaluated for quantitative immunohistochemical and behavioral changes after trauma. P7C3-A20 (10 mg/kg) or vehicle was initiated intraperitoneally 30 min postsurgery and twice per day every day thereafter for 7 days. Administration of P7C3-A20 significantly reduced overall contusion volume, preserved vulnerable anti-neuronal nuclei (NeuN)-positive pericontusional cortical neurons, and improved sensorimotor function 1 week after trauma. P7C3-A20 treatment also significantly increased both bromodeoxyuridine (BrdU)- and doublecortin (DCX)-positive cells within the subgranular zone of the ipsilateral dentate gyrus 1 week after TBI. Five weeks after TBI, animals treated with P7C3-A20 showed significantly increased BrdU/NeuN double-labeled neurons and improved cognitive function in the Morris water maze, compared to TBI-control animals. These results suggest that P7C3-A20 is neuroprotective and promotes endogenous reparative strategies after TBI. We propose that the chemical scaffold represented by P7C3-A20 provides a basis for optimizing and advancing new pharmacological agents for protecting patients against the early and chronic consequences of TBI.[2]
Augmenting hippocampal neurogenesis represents a potential new strategy for treating depression. Here we test this possibility by comparing hippocampal neurogenesis in depression-prone ghrelin receptor (Ghsr)-null mice to that in wild-type littermates and by determining the antidepressant efficacy of the P7C3 class of neuroprotective compounds. Exposure of Ghsr-null mice to chronic social defeat stress (CSDS) elicits more severe depressive-like behavior than in CSDS-exposed wild-type littermates, and exposure of Ghsr-null mice to 60% caloric restriction fails to elicit antidepressant-like behavior. CSDS resulted in more severely reduced cell proliferation and survival in the ventral dentate gyrus (DG) subgranular zone of Ghsr-null mice than in that of wild-type littermates. Also, caloric restriction increased apoptosis of DG subgranular zone cells in Ghsr-null mice, although it had the opposite effect in wild-type littermates. Systemic treatment with P7C3 during CSDS increased survival of proliferating DG cells, which ultimately developed into mature (NeuN+) neurons. Notably, P7C3 exerted a potent antidepressant-like effect in Ghsr-null mice exposed to either CSDS or caloric restriction, while the more highly active analog P7C3-A20 also exerted an antidepressant-like effect in wild-type littermates. Focal ablation of hippocampal stem cells with radiation eliminated this antidepressant effect, further attributing the P7C3 class antidepressant effect to its neuroprotective properties and resultant augmentation of hippocampal neurogenesis. Finally, P7C3-A20 demonstrated greater proneurogenic efficacy than a wide spectrum of currently marketed antidepressant drugs. Taken together, our data confirm the role of aberrant hippocampal neurogenesis in the etiology of depression and suggest that the neuroprotective P7C3-compounds represent a novel strategy for treating patients with this disease.[3] |
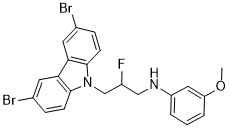
| 分子式 |
C22H19BR2FN2O
|
|---|---|
| 分子量 |
506.20546746254
|
| 精确质量 |
503.984
|
| 元素分析 |
C, 52.20; H, 3.78; Br, 31.57; F, 3.75; N, 5.53; O, 3.16
|
| CAS号 |
1235481-90-9
|
| 相关CAS号 |
P7C3;301353-96-8
|
| PubChem CID |
46853447
|
| 外观&性状 |
Typically exists as White to yellow solids at room temperature
|
| 密度 |
1.6±0.1 g/cm3
|
| 沸点 |
641.3±55.0 °C at 760 mmHg
|
| 闪点 |
341.7±31.5 °C
|
| 蒸汽压 |
0.0±1.9 mmHg at 25°C
|
| 折射率 |
1.647
|
| LogP |
6.96
|
| tPSA |
26.19
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
487
|
| 定义原子立体中心数目 |
0
|
| SMILES |
FC(CNC1=CC(OC)=CC=C1)CN2C3=CC=C(Br)C=C3C4=CC(Br)=CC=C24
|
| InChi Key |
XNLTWMQBJFWQOU-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C22H19Br2FN2O/c1-28-18-4-2-3-17(11-18)26-12-16(25)13-27-21-7-5-14(23)9-19(21)20-10-15(24)6-8-22(20)27/h2-11,16,26H,12-13H2,1H3
|
| 化学名 |
N-[3-(3,6-dibromocarbazol-9-yl)-2-fluoropropyl]-3-methoxyaniline
|
| 别名 |
P7C3-A20; 1235481-90-9; N-(3-(3,6-dibromo-9H-carbazol-9-yl)-2-fluoropropyl)-3-methoxyaniline; N-[3-(3,6-dibromocarbazol-9-yl)-2-fluoropropyl]-3-methoxyaniline; CHEMBL2442625; N-[3-(3,6-dibromo-9H-carbazol-9-yl)-2-fluoropropyl]-3-methoxyaniline; 1235481-90-9 (free base); 9H-Carbazole-9-propanamine, 3,6-dibromo-beta-fluoro-N-(3-methoxyphenyl)-; P7C3A20; P7C3 A20
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 100 mg/mL (~197.55 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 3.85 mg/mL (7.61 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 38.5 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.94 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9755 mL | 9.8773 mL | 19.7546 mL | |
| 5 mM | 0.3951 mL | 1.9755 mL | 3.9509 mL | |
| 10 mM | 0.1975 mL | 0.9877 mL | 1.9755 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。