| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|

| 靶点 |
5-HT3 Receptor
Human 5-HT3A receptors (Ki: 0.15 nM), rat 5-HT3 receptors (Ki: 0.2 nM); no significant binding to other serotonin receptors (5-HT1A, 5-HT2A, 5-HT4) or dopamine D2, muscarinic M1 receptors (Ki > 1000 nM) [1] |
|---|---|
| 体外研究 (In Vitro) |
帕洛诺司琼是一种高效、选择性的第二代 5-HT3 受体拮抗剂,其 5-HT3 受体结合亲和力比其他 5-HT3 受体拮抗剂高约 100 倍(pKi 10.5,而格拉司琼为 8.91) ,托烷司琼为 8.81,昂丹司琼为 8.39,多拉司琼为 7.6)。帕洛诺司琼还具有较长的血浆消除半衰期,约为 40 小时,明显长于同类药物(昂丹司琼,4 小时;托烷司琼,7.3 小时;多拉司琼,7.5 小时;雷尼司琼,8.9 小时)。激酶测定:帕洛诺司琼是一种高效、选择性的第二代 5-HT3 受体拮抗剂,其 5-HT3 受体结合亲和力比其他 5-HT3 受体拮抗剂高约 100 倍(pKi 10.5,而格拉司琼为 8.91,托烷司琼为 8.81,昂丹司琼为 8.39,多拉司琼为 7.6)。细胞测定:帕洛诺司琼是一种 5-HT3 拮抗剂,用于预防和治疗化疗引起的恶心和呕吐 (CINV)。 IC50 值: 目标:5-HT3 受体帕洛诺司琼是控制第一个化疗疗程后 24 小时以上出现的迟发性 CINV 恶心和呕吐最有效的 5-HT3 拮抗剂。
5-HT3受体结合活性:盐酸帕洛诺司琼可竞争性置换人重组5-HT3A受体上的选择性5-HT3配体[³H]昂丹司琼,IC50为0.12 nM;置换大鼠皮层5-HT3受体上[³H]昂丹司琼的IC50为0.18 nM(放射性配体结合实验)[1] - 5-HT3受体功能抑制:在表达内源性5-HT3受体的NG108-15细胞中,盐酸帕洛诺司琼可剂量依赖性抑制5-HT(10 μM)诱导的细胞内钙升高。该抑制作用的IC50为0.3 nM,10 nM时达到最大抑制率(>95%)(钙流实验)[1] - 文献[2](聚焦临床研究)中未描述盐酸帕洛诺司琼的体外活性数据[2] |
| 体内研究 (In Vivo) |
大鼠脑中的定量放射自显影研究表明[3H]-RS 25259-197的5-HT3受体位点的差异分布。在孤束核和后核区可见高密度位点,在三叉神经脊束、齿状回腹侧和基底内侧杏仁核中可见中等密度位点,而在海马CAl、顶叶皮层、中缝和小脑中可见低密度位点。7总之,用放射性标记和非放射性标记的RS 25259-197(S,S对映异构体)进行的功能、结合和分布研究建立了高效选择性5-HT3受体拮抗剂的图谱[1]。
动物模型中的止吐活性(来自[1]): - 顺铂诱导雪貂呕吐:在顺铂(10 mg/kg,静脉注射)给药前30分钟,静脉注射盐酸帕洛诺司琼 0.01 mg/kg、0.1 mg/kg、1 mg/kg,24小时观察期内分别减少约40%、90%、>95%的呕吐次数。0.1 mg/kg剂量还将首次呕吐出现时间从顺铂单独处理组的约2小时延长至约8小时[1] - 5-HT诱导大鼠呕吐:皮下注射盐酸帕洛诺司琼(0.03 mg/kg)可抑制5-HT(2 mg/kg,静脉注射)诱导的呕吐,抑制率约85%[1] - 癌症患者临床止吐疗效(来自[2]): - 急性化疗相关性恶心呕吐(CINV):接受高剂量顺铂(>50 mg/m²)化疗的患者,在化疗前30分钟静脉注射盐酸帕洛诺司琼(0.25 mg),0~24小时内完全缓解率(无呕吐、无解救用药)为60%,显著高于安慰剂组的35%[2] - 延迟性CINV:化疗后24~120小时内,盐酸帕洛诺司琼(0.25 mg)的完全缓解率为50%,显著高于安慰剂组的28%[2] - 中度致吐性化疗(MEC)中的剂量效应:盐酸帕洛诺司琼 0.25 mg与0.5 mg剂量在急性CINV(72% vs. 75%)和延迟性CINV(60% vs. 62%)中的完全缓解率相近,提示高剂量无额外获益[2] |
| 酶活实验 |
帕洛司琼是5-HT3受体的第二代、高选择性、强效拮抗剂,其对受体的结合亲和力约为5-HT3受体其他拮抗剂的100倍(pKi 10.5,而格拉司琼为8.91,托烷司琼为8.81,昂丹司琼为8.39,多拉司琼为7.6)
用四种5-HT3受体配体[3H]-喹帕嗪、[3H]-格拉司琼、[3H]-RS 42348-197和[3H]-RS 25259-197对5-HT3受体进行放射配体结合测定。根据Wong等人(1993a)的方法在Tris-Krebs缓冲液(组成mM:NaCl 154,KCI 5.4,KH2PO4 1.2,CaCI2.5,MgCI2 1.0,Dglucose 11,Tris25,pH7.4,25°C)中制备膜,并在总体积为0.5 ml的25°C下孵育60分钟。用8种浓度的放射性配体进行饱和研究,范围从4 pm到4 nm。用0.1至0.4nM的放射性配体进行竞争研究。非特异性结合是用0.1M(S)-氮氯吡啶定义的。通过在用0.3%聚乙烯亚胺预处理的GF/B过滤器上的真空过滤终止反应。然后用冰冷的0.1M氯化钠洗涤过滤器10 s,干燥并通过液体闪烁光谱法测定过滤器上保留的放射性。在所有研究中,蛋白质浓度通过Biorad比色法测定,以牛丙种球蛋白为标准(Bradford,1976)。通过使用10个浓度的非放射性标记的化合物产生竞争曲线。还测定了[3H]-RS 25259-197和[3H]-格拉司琼的缔合率和离解率[1]。 5-HT3受体结合实验(来自[1]): - 将人重组5-HT3A受体(HEK293细胞表达)或大鼠皮层膜制备物与[³H]昂丹司琼(终浓度0.5 nM)及盐酸帕洛诺司琼(浓度0.01 nM~100 nM)在结合缓冲液(50 mM Tris-HCl pH 7.4、120 mM NaCl、5 mM KCl、0.1% BSA)中混合。混合物在25°C孵育120分钟后,通过预浸泡于0.5%聚乙烯亚胺的玻璃纤维滤膜过滤,分离结合态与游离态配体。滤膜用冰浴结合缓冲液洗涤3次,通过液体闪烁计数器检测放射性,采用Cheng-Prusoff方程计算Ki值[1] - 5-HT3受体功能实验(钙流检测,来自[1]): - 将NG108-15细胞(内源性表达5-HT3受体)在含20 mM HEPES、2 mM CaCl2的HBSS缓冲液中,用钙敏感染料Fluo-4 AM(5 μM)于37°C负载45分钟。细胞洗涤后重悬于HBSS,用盐酸帕洛诺司琼(0.01 nM~100 nM)预处理15分钟,加入5-HT(10 μM)诱导钙升高,通过流式细胞仪检测荧光强度(激发波长488 nm,发射波长525 nm),持续5分钟。根据量效曲线推导抑制5-HT诱导钙流的IC50[1] |
| 细胞实验 |
帕洛诺司琼是一种 5-HT3 拮抗剂,用于治疗和预防化疗 (CINV) 引起的恶心和呕吐。 IC50 值:在 5-HT3 拮抗剂中,5-HT3 受体帕洛诺司琼在治疗迟发性 CINV 恶心和呕吐方面最成功,这种恶心和呕吐在化疗方案初始剂量后 24 小时内出现。
|
| 动物实验 |
Autoradiographical studies[1]
Coronal sections of rat and mouse brains were cut at 20 ,um thickness. Sections were dried and pre-incubated in Tris-HCl buffer (50 mM Tris, 120 mM NaCl, pH 7.4, 22°C) for 30 min. The sections were then covered with the same buffer contain- -4 ing 1.0 nM [3H]-RS 42358-197 or [3H]-RS 25259-197 for 60 min at 22°C. Non-specific binding was defined in the presence of 1.0 tLM (S)-zacopride. The incubations were ter- -n minated by rinsing the slides for two washes of 5 min in ice cold buffer. The sections were dried and apposed, together with 3H polymer standards (Amersham, Inc.) to tritiumsensitive X-ray film for 24 weeks. The autoradiograms were then analysed by digital image analysis with the MCID imaging system (Imaging Research, Inc.). Brain areas were verified on cresyl violet stained sections after autoradiography, using the areas described in the rat brain atlas of Paxinos & Watson (1985). Cisplatin-induced emesis model in ferrets (from [1]): - Male ferrets (1.0–1.5 kg) were fasted for 12 hours before the experiment and randomly divided into 4 groups (n=6/group): vehicle (saline, intravenous), Palonosetron HCl 0.01 mg/kg, 0.1 mg/kg, 1 mg/kg (intravenous). Drugs were administered 30 minutes before cisplatin (10 mg/kg, intravenous). Ferrets were placed in individual cages, and the number of emetic episodes (defined as forceful expulsion of gastric contents) and time to first emesis were recorded for 24 hours. Food and water were provided ad libitum starting 4 hours post-cisplatin [1] - 5-HT-induced emesis model in rats (from [1]): - Male Sprague-Dawley rats (250–300 g) were fasted for 24 hours (water allowed). Rats were divided into 2 groups (n=8/group): vehicle (saline, subcutaneous), Palonosetron HCl 0.03 mg/kg (subcutaneous). Thirty minutes later, 5-HT (2 mg/kg) was injected intravenously. Rats were observed for 1 hour, and the number of emetic episodes was counted [1] - Clinical administration protocol (from [2]): - Adult cancer patients (n=600, aged 18–75 years) receiving cisplatin-based chemotherapy were randomized to 3 groups: Palonosetron HCl 0.25 mg (intravenous), Palonosetron HCl 0.5 mg (intravenous), or placebo. All treatments were administered as a single bolus injection 30 minutes before chemotherapy. Patients were monitored for emetic episodes, use of rescue antiemetics (e.g., metoclopramide), and adverse events for 120 hours post-chemotherapy. Acute CINV was defined as episodes occurring 0–24 hours post-chemotherapy, and delayed CINV as 24–120 hours [2] |
| 药代性质 (ADME/PK) |
Clinical pharmacokinetics (from [2]):
- Intravenous administration of Palonosetron HCl (0.25 mg) in cancer patients: Peak plasma concentration (Cmax) = 3.5 ng/mL, time to Cmax (Tmax) = 5 minutes, elimination half-life (t1/2) = 42 hours, clearance (CL) = 5.8 mL/min, volume of distribution (Vd) = 190 L [2] - Metabolic profile: Palonosetron HCl is metabolized in the liver primarily via CYP2D6 (~50%) and CYP3A4 (~30%); the major metabolite (PAL-103) is inactive (Ki for 5-HT3 receptors > 100 nM) [2] - Excretion: ~80% of the dose is excreted in urine (30% as unchanged drug, 50% as metabolites) over 120 hours; ~10% is excreted in feces [2] - Animal pharmacokinetics (from [1]): - Intravenous administration of Palonosetron HCl (0.1 mg/kg) in rats: t1/2 = 8 hours, CL = 12 mL/min/kg, Vd = 6.5 L/kg [1] - Oral bioavailability in rats: ~35% (after oral administration of 1 mg/kg Palonosetron HCl) [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Clinical toxicity (from [2]):
- Adverse events (AEs) in patients receiving Palonosetron HCl (0.25 mg): The most common AEs were headache (15%), constipation (10%), and fatigue (8%); all were mild to moderate (Grade 1–2) and resolved without intervention [2] - Hepatic/renal safety: No significant changes in serum alanine transaminase (ALT), aspartate transaminase (AST), creatinine, or blood urea nitrogen (BUN) were observed in patients treated with Palonosetron HCl compared to placebo [2] - Animal toxicity (from [1]): - Acute toxicity in mice: Single intravenous dose of Palonosetron HCl up to 50 mg/kg showed no mortality; mice exhibited transient sedation but recovered within 4 hours. Histopathological examination of liver, kidney, and brain revealed no abnormal lesions [1] - Plasma protein binding: In human plasma, Palonosetron HCl has a binding rate of 91% (equilibrium dialysis method); in rat plasma, binding rate is 88% [1][2] - Drug-drug interactions (from [2]): No significant interactions were observed when Palonosetron HCl was co-administered with cisplatin, cyclophosphamide, or dexamethasone (common chemotherapy adjuvants) [2] |
| 参考文献 | |
| 其他信息 |
Palonosetron hydrochloride is a hydrochloride obtained by combining palonosetron with one molar equivalent of hydrogen chloride; an antiemetic used in combination with netupitant (under the trade name Akynzeo) to treat nausea and vomiting in patients undergoing cancer chemotherapy. It has a role as an antiemetic and a serotonergic antagonist. It contains a palonosetron(1+).
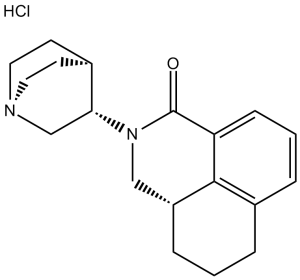
Palonosetron Hydrochloride is the hydrochloride salt of palonosetron, a carbazole derivative and a selective serotonin receptor antagonist with antiemetic activity. Palonosetron competitively blocks the action of serotonin at 5-hydroxytryptamine type 3 (5-HT3) receptors located on vagal afferents in the chemoreceptor trigger zone (CTZ), resulting in suppression of chemotherapy-induced nausea and vomiting. The CTZ is located in the area postrema on the dorsal surface of the medulla oblongata at the caudal end of the fourth ventricle and outside the blood-brain barrier (BBB). Isoquinoline and quinuclidine derivative that acts as a 5-HT3 RECEPTOR antagonist. It is used in the prevention of nausea and vomiting induced by cytotoxic chemotherapy, and for the prevention of post-operative nausea and vomiting. See also: Palonosetron (has active moiety); Fosnetupitant; palonosetron hydrochloride (component of); Netupitant; palonosetron hydrochloride (component of) ... View More ... Drug Indication Aloxi is indicated in adults for: the prevention of acute nausea and vomiting associated with highly emetogenic cancer chemotherapy,the prevention of nausea and vomiting associated with moderately emetogenic cancer chemotherapy. Aloxi is indicated in paediatric patients 1 month of age and older for: the prevention of acute nausea and vomiting associated with highly emetogenic cancer chemotherapy and prevention of nausea and vomiting associated with moderately emetogenic cancer chemotherapy. Palonosetron Hospira is indicated in adults for: the prevention of acute nausea and vomiting associated with highly emetogenic cancer chemotherapy; the prevention of nausea and vomiting associated with moderately emetogenic cancer chemotherapy. Palonosetron Hospira is indicated in paediatric patients 1 month of age and older for: the prevention of acute nausea and vomiting associated with highly emetogenic cancer chemotherapy and prevention of nausea and vomiting associated with moderately emetogenic cancer chemotherapy. Palonosetron HCl is a second-generation, long-acting 5-hydroxytryptamine type 3 (5-HT3) receptor antagonist, chemically designated as (3aS)-2-[(S)-1-Azabicyclo[2.2.2]oct-3-yl]-2,3,3a,4,5,6-hexahydro-1-oxo-1H-benz[de]isoquinoline hydrochloride. It was developed to address limitations of first-generation 5-HT3 antagonists (e.g., short half-life, poor efficacy against delayed CINV) [1][2] - Mechanism of action: Palonosetron HCl competitively binds to 5-HT3 receptors in the peripheral gastrointestinal tract (vagal afferents) and central nervous system (chemoreceptor trigger zone, CTZ), blocking 5-HT-mediated activation of emetic pathways—this is critical for preventing CINV, as chemotherapy-induced intestinal mucosal damage releases 5-HT, which stimulates 5-HT3 receptors [1] - Clinical indication: Approved for the prevention of acute and delayed chemotherapy-induced nausea and vomiting (CINV) in adult cancer patients receiving highly or moderately emetogenic chemotherapy (HEC/MEC). Its long half-life (≈40 hours) allows single-dose administration per chemotherapy cycle, unlike first-generation agents (e.g., ondansetron) which require multiple doses [2] - Literature [2] demonstrated that Palonosetron HCl is superior to placebo in controlling both acute and delayed CINV, with a favorable safety profile |
| 分子式 |
C19H25CLN2O3
|
|---|---|
| 分子量 |
332.87
|
| 精确质量 |
332.165
|
| 元素分析 |
C, 68.56; H, 7.57; Cl, 10.65; N, 8.42; O, 4.81
|
| CAS号 |
135729-62-3
|
| 相关CAS号 |
Palonosetron-d3 hydrochloride; 1246816-81-8; Palonosetron; 135729-61-2; (R,R)-Palonosetron Hydrochloride; 135729-75-8
|
| PubChem CID |
6918303
|
| 外观&性状 |
White to off-white solid powder
|
| 沸点 |
470.4ºC at 760 mmHg
|
| 熔点 |
>290ºC
|
| 闪点 |
209.5ºC
|
| 蒸汽压 |
5.07E-09mmHg at 25°C
|
| LogP |
3.334
|
| tPSA |
23.55
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
23
|
| 分子复杂度/Complexity |
456
|
| 定义原子立体中心数目 |
2
|
| SMILES |
O=C1N(C[C@@]([H])(CCC2)C3=C2C=CC=C13)[C@@H]4CN5CCC4CC5.[H]Cl
|
| InChi Key |
OLDRWYVIKMSFFB-SSPJITILSA-N
|
| InChi Code |
InChI=1S/C19H24N2O.ClH/c22-19-16-6-2-4-14-3-1-5-15(18(14)16)11-21(19)17-12-20-9-7-13(17)8-10-20;/h2,4,6,13,15,17H,1,3,5,7-12H2;1H/t15-,17-;/m1./s1
|
| 化学名 |
(3aS)-2-[(3S)-1-azabicyclo[2.2.2]octan-3-yl]-3a,4,5,6-tetrahydro-3H-benzo[de]isoquinolin-1-one;hydrochloride
|
| 别名 |
RS-25233-197; RS25233-198; RS 25259, RS 25259 197; Palonosetron hydrochloride; RS 25233-197; RS25233-197; RS-25233-198; RS 25233-198; RS-25259-197; US brand name: Aloxi; Akynzeo
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 0.33 mg/mL (0.99 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 3.3 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 0.33 mg/mL (0.99 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 3.3 mg/mL 澄清 DMSO 储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 0.33 mg/mL (0.99 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 100 mg/mL (300.42 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0042 mL | 15.0209 mL | 30.0418 mL | |
| 5 mM | 0.6008 mL | 3.0042 mL | 6.0084 mL | |
| 10 mM | 0.3004 mL | 1.5021 mL | 3.0042 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05956899 | Recruiting | Drug: Palonosetron Drug: Ondansetron |
Idiopathic Scoliosis Postoperative Nausea and Vomiting |
University of Malaya | June 1, 2023 | Phase 4 |
| NCT04507711 | Recruiting | Drug: 0 ul of palonosetron Drug: 1 ul of palonosetron |
Blood Coagulation Disorder | Seoul National University Bundang Hospital |
September 16, 2020 | Not Applicable |
| NCT03817970 | Recruiting | Drug: Granisetron Drug: Palonosetron |
Nephrotoxicity | University of Colorado, Denver | November 15, 2019 | Phase 3 |
| NCT05199818 | Recruiting | Drug: Palonosetron HCl Buccal Film 0.5 mg Drug: IV Palonosetron 0.25 mg |
Chemotherapy-induced Nausea and Vomiting |
Xiamen LP Pharmaceutical Co., Ltd |
March 1, 2022 | Phase 3 |
| NCT05841849 | Not yet recruiting | Drug: Aprepitant Drug: Palonosetron |
Breast Cancer Chemotherapy-induced Nausea and Vomiting |
Second Affiliated Hospital, School of Medicine, Zhejiang University |
July 2023 | Phase 4 |
 |
|---|
 |
 |