| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
5-HT3 Receptor
In vitro activity: Palonosetron is a second-generation, highly selective, potent antagonist of the 5-HT3 receptor with a binding affinity for the receptor that is approximately 100 times higher than that of other antagonists of the 5-HT3 receptor (pKi 10.5 compared with 8.91 for granisetron, 8.81 for tropisetron, 8.39 for ondansetron, and 7.6 for dolasetron). Additionally, palonosetron has an extended plasma elimination half-life of about 40 hours, which is substantially longer than that of other drugs in its class (ranisetron, 8.9 hours; tropisetron, 7.3 hours; dolasetron, 7.5 hours). |
|---|---|
| 体外研究 (In Vitro) |
体外活性:帕洛诺司琼是一种高效、选择性的第二代 5-HT3 受体拮抗剂,其 5-HT3 受体结合亲和力比其他 5-HT3 受体拮抗剂高约 100 倍(pKi 10.5,而格拉司琼为 8.91) ,托烷司琼为 8.81,昂丹司琼为 8.39,多拉司琼为 7.6)。帕洛诺司琼还具有较长的血浆消除半衰期,约为 40 小时,明显长于同类药物(昂丹司琼,4 小时;托烷司琼,7.3 小时;多拉司琼,7.5 小时;雷尼司琼,8.9 小时)。激酶测定:帕洛诺司琼是一种高效、选择性的第二代 5-HT3 受体拮抗剂,其 5-HT3 受体结合亲和力比其他 5-HT3 受体拮抗剂高约 100 倍(pKi 10.5,而格拉司琼为 8.91,托烷司琼为 8.81,昂丹司琼为 8.39,多拉司琼为 7.6)。细胞测定:帕洛诺司琼是一种 5-HT3 拮抗剂,用于预防和治疗化疗引起的恶心和呕吐 (CINV)。 IC50 值: 目标:5-HT3 受体帕洛诺司琼是控制第一个化疗疗程后 24 小时以上出现的迟发性 CINV 恶心和呕吐最有效的 5-HT3 拮抗剂。
在豚鼠离体回肠中,RS 25259-198 拮抗了5-HT引起的收缩反应。其表观功能亲和力(pKB)为6.7 ± 0.1。 [2] 在使用NG-108-15细胞膜和[³H]-喹吡嗪进行的放射性配体结合实验中,RS 25259-198 表现出结合亲和力(pKi)为8.40 ± 0.07,希尔系数(nH)为0.89 ± 0.08。 [2] |
| 体内研究 (In Vivo) |
大鼠脑中的定量放射自显影研究表明[3H]-RS 25259-197的5-HT3受体位点的差异分布。在孤束核和后核区可见高密度位点,在三叉神经脊束、齿状回腹侧和基底内侧杏仁核中可见中等密度位点,而在21]。
在一项涉及563名接受中致吐性化疗的成年癌症患者的III期随机双盲研究中,单次静脉注射帕洛诺司琼 0.25 mg在预防急性(0–24小时)、延迟性(24–120小时)和总体(0–120小时)化疗引起的恶心呕吐(CINV)方面显著优于单次静脉注射昂丹司琼32 mg。完全缓解率(无呕吐发作且未使用解救药物)分别为:急性期:81.0% vs 68.6% (P<0.01);延迟期:74.1% vs 55.1% (P<0.001);总体:69.3% vs 50.3% (P<0.001)。[1] 在延迟期(66.7% vs 50.3%;P=0.001)和总体期(63.0% vs 44.9%;P=0.001),帕洛诺司琼 0.25 mg的完全控制率(无呕吐发作、无需解救药物且恶心程度不超过轻度)也显著高于昂丹司琼。[1] 使用帕洛诺司琼 0.25 mg治疗至治疗失败(首次呕吐发作或首次使用解救药物)的时间显著长于昂丹司琼 (P<0.001)。帕洛诺司琼0.25 mg组的第一四分位治疗失败时间(46.5小时)是昂丹司琼组(19.5小时)的两倍多。[1] 在急性期、延迟期和总体期的呕吐发作次数、无呕吐发作患者比例以及第3-5天无恶心患者比例方面,帕洛诺司琼 0.25 mg consistently优于昂丹司琼。[1] |
| 酶活实验 |
帕洛诺司琼是第二代、高选择性、强效的 5-HT3 受体拮抗剂,与该受体的结合亲和力比其他 5-HT3 受体拮抗剂高约 100 倍(pKi 10.5,而格拉司琼为 8.91) ,托烷司琼为 8.81,昂丹司琼为 8.39,多拉司琼为 7.6)。
豚鼠回肠收缩力实验: 将近端回肠段(2厘米)悬置于含有甲麦角新碱(用于阻断5-HT1/5-HT2受体)和5-甲氧基色胺(用于使5-HT1P受体脱敏)的台氏液中,在37°C下承受1g静息张力。在拮抗剂存在和不存在的情况下,经过60分钟平衡期后,构建5-HT(10 nM – 10 μM)的非累积浓度-反应曲线。表观亲和力(pKB)通过浓度比位移计算得出。 [2] [³H]-喹吡嗪结合实验: 制备膜并在Tris-Krebs缓冲液中于25°C孵育60分钟。饱和研究使用了八种浓度的[³H]-喹吡嗪(4 pM 至 4 nM)。竞争性研究使用0.1至0.4 nM的放射性配体和10种浓度的未标记化合物。非特异性结合用0.1 μM (S)-扎考必利定义。反应通过在用0.3%聚乙烯亚胺预处理过的GF/B过滤器上进行真空过滤来终止,随后用冰凉的0.1 M NaCl洗涤。结合的放射性通过液体闪烁光谱法测定。结合数据使用四参数逻辑方程和Cheng-Prusoff校正进行分析。 [2] |
| 细胞实验 |
帕洛诺司琼是一种 5-HT3 拮抗剂,用于治疗和预防化疗 (CINV) 引起的恶心和呕吐。 IC50 值:在 5-HT3 拮抗剂中,5-HT3 受体帕洛诺司琼在治疗迟发性 CINV 恶心和呕吐方面最成功,这种恶心和呕吐在化疗方案初始剂量后 24 小时内出现。
|
| 动物实验 |
Autoradiographical studies[2]
Coronal sections of rat and mouse brains were cut at 20 ,um thickness. Sections were dried and pre-incubated in Tris-HCl buffer (50 mM Tris, 120 mM NaCl, pH 7.4, 22°C) for 30 min. The sections were then covered with the same buffer contain- -4 ing 1.0 nM [3H]-RS 42358-197 or [3H]-RS 25259-197 for 60 min at 22°C. Non-specific binding was defined in the presence of 1.0 tLM (S)-zacopride. The incubations were ter- -n minated by rinsing the slides for two washes of 5 min in ice cold buffer. The sections were dried and apposed, together with 3H polymer standards (Amersham, Inc.) to tritiumsensitive X-ray film for 24 weeks. The autoradiograms were then analysed by digital image analysis with the MCID imaging system (Imaging Research, Inc.). Brain areas were verified on cresyl violet stained sections after autoradiography, using the areas described in the rat brain atlas of Paxinos & Watson (1985). |
| 药代性质 (ADME/PK) |
Palonosetron has an extended plasma elimination half-life of approximately 40 hours, which is significantly longer than other 5-HT3 receptor antagonists (e.g., ondansetron: ~4 h; tropisetron: ~7.3 h; dolasetron: ~7.5 h; granisetron: ~8.9 h). [1]
|
| 毒性/毒理 (Toxicokinetics/TK) |
In the Phase III clinical trial, palonosetron was well tolerated. The most common treatment-related adverse event (adverse reaction) was headache, reported in 4.8% of patients receiving palonosetron 0.25 mg and 5.3% receiving palonosetron 0.75 mg (compared to 5.3% with ondansetron). [1]
Other treatment-related adverse events reported in >2% of patients included constipation (1.6% for palonosetron 0.25 mg, 3.2% for 0.75 mg) and dizziness (0.5% for 0.25 mg, 3.2% for ondansetron). [1] No significant treatment-related changes were observed in laboratory parameters, vital signs, or electrocardiogram (ECG) recordings. The mean post-dose change from baseline in QTc (Fridericia correction) was 1 ms for palonosetron 0.25 mg and 2 ms for palonosetron 0.75 mg. [1] Most adverse events were assessed as related to the patient's underlying cancer or chemotherapy, not to the study medication. No significant safety concerns were raised. [1] |
| 参考文献 | |
| 其他信息 |
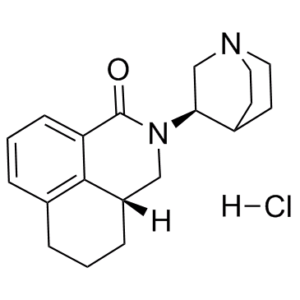
RS 25259-198 is one of the four enantiomers of the isoquinoline compound RS 25259-197. Its full chemical name is (3aR)-2-[(1R)-1-azabicyclo[2.2.2]oct-3-yl]-2,3,3a,4,5,6-hexahydro-1-oxo-1H-benz[de]isoquinoline hydrochloride. [2]
Among the four enantiomers tested, RS 25259-198 (R,R) exhibited the lowest apparent affinity (pKB = 6.7) for 5-HT₃ receptors mediating contraction in guinea-pig isolated ileum, compared to the (S,S) enantiomer (pKB = 8.8). [2] RS 25259-198 is a highly selective 5-HT₃ receptor antagonist, as it showed low affinity (pKi < 6.0) at 28 other neurotransmitter receptors and ion channels in binding assays, including adrenoceptors, muscarinic, dopamine, opioid, and other 5-HT receptor subtypes. [2] Palonosetron is a novel second-generation 5-HT3 receptor antagonist developed to improve the prevention of both acute and delayed CINV, an area where first-generation 5-HT3 antagonists have limited efficacy. [1] The prolonged antiemetic efficacy of a single dose of palonosetron is likely related to its high receptor binding affinity and long plasma half-life. [1] The doses selected for the Phase III study (0.25 mg and 0.75 mg) were based on a prior Phase II dose-finding study which identified 0.25 mg (~3.0 µg/kg) as the minimum effective dose for preventing CINV after highly emetogenic chemotherapy. [1] This study demonstrated that a single intravenous dose of palonosetron 0.25 mg provides superior and prolonged protection against nausea and vomiting compared to ondansetron in patients receiving moderately emetogenic chemotherapy. [1] |
| 分子式 |
C19H24N2O.HCL
|
|---|---|
| 分子量 |
332.87
|
| 精确质量 |
332.17
|
| 元素分析 |
C, 76.99; H, 8.16; N, 9.45; O, 5.40
|
| CAS号 |
135729-75-8
|
| 相关CAS号 |
Palonosetron hydrochloride; 135729-62-3; Palonosetron; 135729-61-2
|
| PubChem CID |
18651160
|
| 外观&性状 |
Solid
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
470.4±45.0 °C at 760 mmHg
|
| 闪点 |
209.5±21.1 °C
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
| 折射率 |
1.646
|
| LogP |
2.61
|
| tPSA |
23.55
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
23
|
| 分子复杂度/Complexity |
456
|
| 定义原子立体中心数目 |
2
|
| SMILES |
O=C1N(C[C@]([H])(CCC2)C3=C2C=CC=C13)[C@H]4CN5CCC4CC5.[H]Cl
|
| InChi Key |
OLDRWYVIKMSFFB-NBLXOJGSSA-N
|
| InChi Code |
InChI=1S/C19H24N2O.ClH/c22-19-16-6-2-4-14-3-1-5-15(18(14)16)11-21(19)17-12-20-9-7-13(17)8-10-20;/h2,4,6,13,15,17H,1,3,5,7-12H2;1H/t15-,17-;/m0./s1
|
| 化学名 |
(3aR)-2-[(3R)-1-azabicyclo[2.2.2]octan-3-yl]-3a,4,5,6-tetrahydro-3H-benzo[de]isoquinolin-1-one;hydrochloride
|
| 别名 |
(R,R)-RS 25259, RS-25233-197; RS25233-198; RS 25259 197; RS 25233-197; (R,R)-RS25233-197; RS-25233-198; RS 25233-198; RS-25259-197; (R,R)-Palonosetron hydrochloride; US brand name: Aloxi and Akynzeo
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0042 mL | 15.0209 mL | 30.0418 mL | |
| 5 mM | 0.6008 mL | 3.0042 mL | 6.0084 mL | |
| 10 mM | 0.3004 mL | 1.5021 mL | 3.0042 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05690802 | Recruiting | Drug: Palonosetron hydrochloride capsules |
Nausea and Vomiting | Xijing Hospital | May 16, 2022 | Not Applicable |
| NCT01370408 | Completed | Drug: Palonosetron Drug: ondansetron |
Chemotherapy-induced Nausea and Vomiting |
Northside Hospital, Inc. | February 2012 | Phase 2 |
| NCT03817970 | Recruiting | Drug: Palonosetron Drug: Ondansetron |
Nephrotoxicity | University of Colorado, Denver | November 15, 2019 | Phase 3 |