| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
FGFR1 (IC50 = 25 nM); VEGFR2 (IC50 = 100 nM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:PD173074 是 FGFR1 的 ATP 竞争性抑制剂,Ki 约为 40 nM。 PD173074 也是 VEGFR2 的有效抑制剂。与 FGFR1 相比,PD173074 弱抑制 Src、InsR、EGFR、PDGFR、MEK 和 PKC 的活性,IC50 值是 1000 倍或更高。 PD173074 以剂量依赖性方式抑制 FGFR1 和 VEGFR2 的自身磷酸化,IC50 分别为 1-5 nM 和 100-200 nM。 PD173074 以剂量依赖性方式抑制 FGF-2 对颗粒神经元存活的促进作用,IC50 为 12 nM,效力比 SU 5402 高 1,000 倍。PD173074 特异性抑制 FGF-2 介导的增殖、分化和 MAPK 效应少突胶质细胞 (OL) 谱系细胞的激活。 PD173074 对多发性骨髓瘤 (MM) 细胞系中的 WT 受体和 FGFR3 突变具有活性。 PD173074 还以剂量依赖性方式有效抑制 FGFR3 的自身磷酸化,IC50 约为 5 nM。 PD173074 治疗可有效降低表达 FGFR3 的 KMS11 和 KMS18 细胞的活力,IC50 <20 nM。 PD173074 对 aFGF 刺激的 MM 细胞生长的抑制与 FGFR3 的表达高度相关。 PD173074 治疗完全消除了 Y373C FGFR3 介导的 NIH 3T3 转化,但不消除 Ras V12 介导的 NIH 3T3 转化,表明 PD173074 特异性靶向 FGFR3 介导的细胞转化,并且缺乏非特异性细胞毒性作用。 PD173074 还诱导 KMS11 和 KMS18 细胞的功能成熟。激酶测定:使用全长 FGFR-1 激酶进行测定,总体积为 100 μL,含有 25 mM HEPES 缓冲液 (pH 7.4)、150 mM NaCl、10 mM MnCl2、0.2 mM 原钒酸钠,浓度为 750 μg/mL谷氨酸和酪氨酸 (4:1) 的无规共聚物、不同浓度的 PD173074 和 60 至 75 ng 的酶。通过添加 [γ-32P]ATP(每次孵育 5 μM ATP,含有 0.4 μCi [γ-32P]ATP)启动反应,并将样品在 25°C 下孵育 10 分钟。通过添加 30% 三氯乙酸并将材料沉淀到玻璃纤维过滤垫上来终止反应。用15%三氯乙酸洗涤过滤器三次,并通过在Wallac 1250 beta板读数器中计算过滤器上保留的放射性来确定[32P]掺入谷氨酸酪氨酸聚合物底物中。非特异性活性定义为在不加酶的情况下孵育样品后保留在过滤器上的放射性。比活性确定为总活性(酶加缓冲液)减去非比活性。以图形方式确定抑制 FGFR-1 酶活性 50% (IC50) 的 PD173074 浓度。细胞分析:PD 173074 剂量依赖性地抑制 FGFR1 的自身磷酸化,IC50 值在 1 ~ 5 nM 范围内。此外,PD 173074 还能抑制 VEGFR2 的自身磷酸化,IC50 值为 100 ~ 200 nM。在 aFGF/肝素存在下,将细胞(KMS11 和 KMS18)与浓度不断增加的 PD173074 一起孵育 48 小时。通过MTT测定活细胞的百分比。
|
| 体内研究 (In Vivo) |
在小鼠中给予 1 mg/kg/天或 2 mg/ka/天的 PD173074 可以以剂量依赖性方式有效阻断 FGF 或 VEGF 诱导的血管生成,且无明显毒性。 PD173074 抑制突变型 FGFR3 转染的 NIH 3T3 细胞在裸鼠体内的生长。 PD173074 抑制 FGFR3 可延迟 KMS11 异种移植骨髓瘤模型中的肿瘤生长并提高小鼠的存活率。在 H-510 异种移植物中,口服 PD173074 可阻止肿瘤生长,与单药顺铂给药相似,与对照假治疗动物相比,增加了中位生存期。在 H-69 异种移植物中,PD173074 在 50% 的小鼠中诱导持续超过 6 个月的完全反应。这些效应与切除肿瘤中细胞凋亡的增加相关,但不是肿瘤脉管系统破坏的结果。
口服PD173074可抑制H510和H69肿瘤的生长,并增强顺铂在裸鼠体内的作用。[5] [18F]FLT-PET是体内对PD173074反应的早期预测因子[5] 为了确定我们是否可以使用适用于临床患者的体内成像技术预测对PD173074的反应,我们接下来使用[18F]FLT-PET监测肿瘤内增殖。在颈部携带皮下H69异种移植物的动物每天口服含或不含PD173074的稀释剂,并在第8天和第14天成像前注射[18F]FLT-PET。图5A显示了一只对照组和一只PD173074治疗动物在治疗前和治疗14天后的代表性[18F]FLT-PET成像。通过分数保留时间对[18F]FLT-PET结果的分析表明,PD173074给药减少了细胞增殖(图5B),分数保留时间是一个与肿瘤大小无关、对灌注依赖性较小的参数。在相同的肿瘤中,通过卡尺测量显示生长抑制(图5A,底部)。这表明[18F]FLT-PET可能提供了一种非侵入性的方法来预测用PD173074等药物治疗的患者的早期肿瘤反应。 |
| 酶活实验 |
在使用全长 FGFR-1 激酶的测定中,使用的总体积为 100 μL。它含有以下浓度:750 μg/mL 谷氨酸和酪氨酸无规共聚物 (4:1)、不同浓度的 PD173074、60 至 75 ng 酶、150 mM NaCl、10 mM MnCl2< /sub>,0.2 mM 原钒酸钠。添加[γ-32P]ATP(每次孵育5 μM ATP,含有0.4 μCi的[γ-32P]ATP)开始反应,并孵育样品在 25°C 下保持 10 分钟。添加百分之三十的三氯乙酸以终止反应,并且材料沉淀到玻璃纤维过滤垫上。用 15% 三氯乙酸清洗过滤器 3 次后,通过计算 Wallac 1250 beta 读板器中过滤器上保留的放射性来测量 [32P] 掺入谷氨酸酪氨酸聚合物底物中。孵育不含酶的样品后,残留在过滤器上的放射性称为非特异性活性。总活性(酶加缓冲液)减去非特异性活性是计算比活性的公式。 IC50图表用于计算抑制FGFR-1酶活性50%的PD173074的浓度。
|
| 细胞实验 |
先前已有过表达 VEGFR2 (Flk-1) 的 NIH 3T3 细胞系的描述。此外,该细胞系天然表达 FGFR1。将在 10% 小牛血清增强的 DMEM 中生长的 1×106 细胞接种到 10 cm2 平板中,并培养 48 小时。然后将细胞放入饥饿培养基(含 0.1% 小牛血清的 DMEM)中,在除去培养基后使其静止。将在饥饿培养基中制备的PD 173074以不同浓度添加到细胞中,并在18小时后孵育5分钟。接下来,在 37°C 下用生长因子 [100 ng/mL VEGF 或 100 ng/mL aFGF 和 10 µg/mL 肝素] 刺激细胞 5 分钟。冰冷 PBS 洗涤后,将细胞溶解在 1 mL 裂解缓冲液中,该缓冲液含有磷酸酶抑制剂 (0.2 mM Na3VO4) 和 25 mM HEPES pH 7.5、150 mM NaCl、1% Triton X-100、10% 甘油、1 mM EGTA、1.5 mM MgCl2、1 mM PMSF、10 µg/mL 抑质素和 10 µg/mL 亮肽素。使用 FGFR1 抗体对细胞裂解物进行免疫沉淀,进行 FGFR1 抑制研究。然后使用磷酸酪氨酸特异性抗体进行 SDS-PAGE 和免疫印迹分析。使用磷酸酪氨酸特异性抗体对细胞裂解物 (20 µL) 进行直接 SDS-PAGE 分析和免疫印迹,以研究 VEGFR2 的抑制作用。
|
| 动物实验 |
Subcutaneous inoculation with 3×105 NIH 3T3 cells expressing Y373C FGFR3 and Ras V12 is performed on six-week-old athymic nude mice. The intraperitoneal injection of 0.05 mol/L lactic acid buffer or 20 mg/kg PD173074 is started on the day of the tumor injection and is administered for nine days. For every experiment, ten mice are used.
Xenografts and immunohistochemistry[5] H510 (1:1 cell suspension; Matrigel) or H69 cells were implanted into the flank of nude mice. When tumors became measurable, 50 mg/kg PD173074/mice or equivalent volume of buffer alone were administered daily for 14 or 28 d. In addition, mice received or did not receive two doses of 5 mg/kg cisplatin. Tumor volume was monitored using a calliper. Animals were sacrificed when tumor burden reached 15 mm in any dimension and survival recorded as a Kaplan-Meier plot. Tissues were formalin fixed and paraffin embedded before staining as indicated in the figure legends. For the endomucin experiments, pictures were acquired using a ×10 objective and analyzed using ImageJ. For activated Caspase 3 and cytokeratin 18 scoring, the number of positive cells in five high-power field views/tumor (five tumors per condition) was determined and results represented as bar graphs (Fig. 5C , bottom). The total number of nuclei per field was determined by manual counting using event flagging in Metamorph. Nuclei partly outside the field of view were excluded. [18F]FLT-PET imaging[5] Animals with subcutaneous H-69 xenografts in the neck were used when the tumors reached ∼150 mm3. The tumor-bearing mice were given vehicle or PD173074 once daily by oral gavage and imaged with [18F]FLT-PET on days 0, 7, and 14 of treatment. Dynamic [18F]FLT-PET studies were carried out on a dedicated small animal PET scanner, quad-HIDAC (Oxford Positron Systems; ref. 15). Scanning was performed as previously described (16). [18F]FLT (80–100 μCi; 2.96–3.7 MBq) was injected into the tail veins of anesthetized mice positioned prone within the scanner. Dynamic scans were acquired in list-mode format over a 60-min period and sorted into 0.5-mm sinogram bins and 19 time frames (0.5 × 0.5 × 0.5 mm voxels; 4 × 15 s, 4 × 60 s, and 11 × 300 s) for image reconstruction. Cumulative images comprising of 30 to 60 min of the dynamic data were used for visualization of radiotracer uptake and to draw regions of interest. Regions of interest were defined on five tumor and five heart slices (each was 0.5 mm thick). Dynamic data from these slices were averaged for each tissue and at each of the 19 time points to obtain time versus radioactivity curves for these tissues. Tumor radioactivity was corrected for physical decay and normalized to that of heart to obtain a standardized uptake value. The fractional retention of tracer was calculated as the normalized uptake in tumors 60 min relative to that at 1.5 min |
| 参考文献 | |
| 其他信息 |
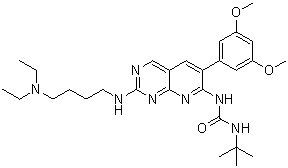
PD173074 is a member of the class of ureas that is 1-tert-butylurea in which one of the hydrogens attached to N(3) is substituted by a pyrido[2,3-d]pyrimidin-7-yl group, which is itself substituted at positions 2 and 6 by a 4-(diethylamino)butyl]amino group and a 3,5-dimethoxyphenyl group, respectively. It is a FGF/VEGF receptor tyrosine kinase inhibitor. It has a role as a fibroblast growth factor receptor antagonist, an antineoplastic agent and an EC 2.7.10.1 (receptor protein-tyrosine kinase) inhibitor. It is a pyridopyrimidine, a member of ureas, a tertiary amino compound, a dimethoxybenzene, an aromatic amine and a biaryl. It is functionally related to a PD-166866.
Angiogenesis, the sprouting of new blood vessels from pre-existing ones, is an essential physiological process in development, yet also plays a major role in the progression of human diseases such as diabetic retinopathy, atherosclerosis and cancer. The effects of the most potent angiogenic factors, vascular endothelial growth factor (VEGF), angiopoietin and fibroblast growth factor (FGF) are mediated through cell surface receptors that possess intrinsic protein tyrosine kinase activity. In this report, we describe a synthetic compound of the pyrido[2,3-d]pyrimidine class, designated PD 173074, that selectively inhibits the tyrosine kinase activities of the FGF and VEGF receptors. We show that systemic administration of PD 173074 in mice can effectively block angiogenesis induced by either FGF or VEGF with no apparent toxicity. To elucidate the determinants of selectivity, we have determined the crystal structure of PD 173074 in complex with the tyrosine kinase domain of FGF receptor 1 at 2.5 A resolution. A high degree of surface complementarity between PD 173074 and the hydrophobic, ATP-binding pocket of FGF receptor 1 underlies the potency and selectivity of this inhibitor. PD 173074 is thus a promising candidate for a therapeutic angiogenesis inhibitor to be used in the treatment of cancer and other diseases whose progression is dependent upon new blood vessel formation. [1] Basic fibroblast growth factor (FGF-2) promotes survival and/or neurite outgrowth from a variety of neurons in cell culture and regenerative processes in vivo. FGFs exert their effects by activating cell surface receptor tyrosine kinases. FGF receptor (FGFR) inhibitors have not been characterized on neuronal cell behaviors to date. In the present study, we show that the FGFR1 inhibitor PD 173074 potently and selectively antagonized the neurotrophic and neurotropic actions of FGF-2. Nanomolar concentrations of PD 173074 prevented FGF-2, but not insulin-like growth factor-1, support of cerebellar granule neuron survival under conditions of serum/K(+) deprivation; another FGF-2 inhibitor, SU 5402, was effective only at a 1,000-fold greater concentration. Neither PD 173074 nor SU 5402, at 100 times their IC(50) values, interfered with the survival of dorsal root ganglion neurons promoted by nerve growth factor, ciliary neurotrophic factor, or glial cell line-derived neurotrophic factor. PD 173074 and SU 5402 displayed 1,000-fold differential IC(50) values for inhibition of FGF-2-stimulated neurite outgrowth in PC12 cells and in granule neurons, and FGF-2-induced mitogen-activated protein kinase (p44/42) phosphorylation. The two inhibitors failed to disturb downstream signalling stimuli of FGF-2. PD 173074 represents a valuable tool for dissecting the role of FGF-2 in normal and pathological nervous system function without compromising the actions of other neurotrophic factors. [2] Multiple studies have shown that migration, proliferation, and differentiation of oligodendrocyte (OL) lineage cells are influenced by fibroblast growth factor-2 (FGF-2) signaling through its receptors (FGFR) FGFR-1, FGFR-2, and FGFR-3. We report the effectiveness and specificity of a unique inhibitor, PD173074, for inhibiting FGF receptor signaling in OL-lineage cells. Three FGF-mediated responses of OL progenitors and two of differentiated OLs were examined by immunofluorescence microscopy and immunoblotting. PD173074 effectively antagonized the effect of FGF-2 on proliferation and differentiation of OL progenitors in culture. One dose of PD173074 at nanomolar concentrations was sufficient to inhibit ongoing FGF-2 mediated proliferation for prolonged periods, in a non-toxic, dose-dependent manner. In contrast, platelet-derived growth factor (PDGF)-induced proliferation was unaffected by PD173074. Similarly, mitogen-activated protein kinase (MAPK) activation, a downstream event after activation of either FGFR or PDGFR, was also blocked by PD173074 in OL progenitors stimulated with FGF-2 but not PDGF. A general tyrosine kinase inhibitor (PD166285), however, antagonized both FGF-2- and PDGF-mediated responses. PD173074 also completely antagonized two phenotypic alterations of differentiated OLs, specifically downregulation of myelin proteins, and their re-entry into the cell cycle. We conclude that PD173704 is an effective and specific inhibitor for multiple FGF-2-mediated responses of both OL progenitors and differentiated OLs. This inhibitor provides a direct approach for identifying the importance of FGF signaling, comparable in effect to a knockout of all FGF receptors and all FGF ligands, while leaving other pathways unaffected. Thus, PD173704 is an excellent tool for investigating the role of FGF signaling in vivo in the context of combinatorial interactions of other signals. [3] |
| 分子式 |
C28H41N7O3
|
|
|---|---|---|
| 分子量 |
523.67
|
|
| 精确质量 |
523.327
|
|
| 元素分析 |
C, 64.22; H, 7.89; N, 18.72; O, 9.17
|
|
| CAS号 |
219580-11-7
|
|
| 相关CAS号 |
|
|
| PubChem CID |
1401
|
|
| 外观&性状 |
Off-white to yellow solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 熔点 |
82-85°C
|
|
| 折射率 |
1.599
|
|
| LogP |
3.33
|
|
| tPSA |
113.53
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
8
|
|
| 可旋转键数目(RBC) |
13
|
|
| 重原子数目 |
38
|
|
| 分子复杂度/Complexity |
690
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O=C(NC1=NC2=NC(NCCCCN(CC)CC)=NC=C2C=C1C3=CC(OC)=CC(OC)=C3)NC(C)(C)C
|
|
| InChi Key |
DXCUKNQANPLTEJ-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C28H41N7O3/c1-8-35(9-2)13-11-10-12-29-26-30-18-20-16-23(19-14-21(37-6)17-22(15-19)38-7)25(31-24(20)32-26)33-27(36)34-28(3,4)5/h14-18H,8-13H2,1-7H3,(H3,29,30,31,32,33,34,36)
|
|
| 化学名 |
1-tert-butyl-3-[2-[4-(diethylamino)butylamino]-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]urea
|
|
| 别名 |
PD 173074; PD-173074; 219580-11-7; 1-(tert-Butyl)-3-(2-((4-(diethylamino)butyl)amino)-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl)urea; 1-tert-butyl-3-[2-[4-(diethylamino)butylamino]-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]urea; MFCD08705327; 1-tert-butyl-3-[2-{[4-(diethylamino)butyl]amino}-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]urea; PD173074
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.77 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.77 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (3.97 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 5% DMSO+corn oil: 15mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9096 mL | 9.5480 mL | 19.0960 mL | |
| 5 mM | 0.3819 mL | 1.9096 mL | 3.8192 mL | |
| 10 mM | 0.1910 mL | 0.9548 mL | 1.9096 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
The expression of MYC and FGFR3 was analyzed by Western blotting in lysates from MGH‐U3 and RT112 cells transfected for 72h withMYCsiRNAs.
TheMYCaccumulation induced by activatedFGFR3 confers sensitivity toBETbromodomain inhibitors inFGFR3‐dependent bladder cancer cellsinvitroandinvivo.EMBO Mol Med.2018 Apr;10(4). pii: e8163. |
MGH‐U3 and RT112 cells were treated with control DMSO, PD [PD173074 (FGFR inhibitor)], SB [SB203580 (p38 inhibitor)] or LY [LY294002 (PI3 kinase inhibitor)] for 72h and cell viability was then assessed by measuring MTT incorporation.
Venn diagram showing the number of upstream regulators (transcription factors) significantly predicted by Ingenuity Pathway Analysis to be involved in the regulation of gene expression observed afterFGFR3knockdown in RT112 and MGH‐U3 cells (left panel).EMBO Mol Med.2018 Apr;10(4). pii: e8163.
|
TheMYCaccumulation induced by activatedFGFR3 is dependent on the activation of p38 andAKT.
MYCandFGFR3 are involved in a positive feedback loop in bladder cancer cell lines expressing an activated form ofFGFR3.EMBO Mol Med.2018 Apr;10(4). pii: e8163. |