| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Poorly absorbed through the skin. Permethrin is rapidly metabolized by ester hydrolysis to inactive metabolites which are excreted primarily in the urine. Lactating cows (three/group) fed permethrin at dose levels of 0, 0.2, 1.0, 10, 50 mg/kg diet for 28 days showed no mortality, and growth and milk production were normal. Permethrin residues were observed in the milk within 3 days at the two highest dietary levels; levels appeared to reach a plateau rapidly and not to increase with time. Analysis of individual cis and trans isomers showed that the ratio of permethrin isomers in milk appeared to change during the course of the study with the cis isomer predominating. Permethrin residues were not found in the tissues of animals that received doses of 1 mg/kg or less. At dose levels of 10 or 50 mg/kg, residues were detected in the tissues, predominantly in the fat. Low levels were also present in the muscle and kidney at the highest dose level. Permethrin did not appear to accumulate in the fat but to reach a plateau rapidly. (14)C-cis-Permethrin was applied to the clipped skin of mice at a level of 1 mg/kg body weight in 0.1 mL of acetone. The mice were restrained until the solvent had evaporated and then placed in mouse metabolism cages. They were sacrificed at 1, 5, 15, 50, 480, and 2880 min after treatment and examined for absorption, distribution, and excretion of the insecticide. About 40% of the applied permethrin had moved from the site of application within 5 min and appeared to move rapidly to other parts of the body. When ten consecutive oral doses of (14)C-trans- or (14)C-cis- permethrin (labelled in the acid or alcohol moieties) at 0.2-0.3 mg/kg bw/day were given to lactating goats, they excreted 72-79% and 25-36% of the trans and cis isomer doses, respectively, in urine & 12-15%, respectively, in the feces. The amounts of the radiocarbon appearing in the milk were <0.7% with any one of the four (14)C-labelled preparations. Concerning the tissue residues 24 hr after the last dose, detectable levels of radiocarbon were found in most tissue, but none was >0.04 mg/kg for the trans isomer or 0.25 mg/kg for the cis isomer. Two human volunteers, who consumed about 2 and 4 mg of permethrin (25:75), respectively, excreted 18-37% and 32-39% of the administered dose, detected as the metabolite Cl2CA, after acid hydrolysis of their urine collected over 24 hr. For more Absorption, Distribution and Excretion (Complete) data for PERMETHRIN (37 total), please visit the HSDB record page. Metabolism / Metabolites Rapidly metabolized by ester hydrolysis to inactive metabolites which are excreted primarily in the urine. When the four (14)C-preparations of (IRS)-trans-, (IR)-trans- , (IRS)-cis, and (IR)-cis-permethrin labeled in the alcohol and acid moieties were administered orally to male rats at 1.6-4.8 mg/kg, the compounds were rapidly metabolized and the (14)C from the acid and alcohol moeity was almost completely eliminated from the body within a few days. ... The major metabolic reactions of both permethrin isomers /(trans and cis)/ were oxidation at the trans and cis portions of the gem-dimethyl group of the acid moiety and at the 2'- and 4'-positions of the alcohol moiety, ester cleavage, and the conjugation of the resulting carboxylic acids, alcohols, and phenols with glucuronic acid, glycine, and sulfuric acid. The cis isomer is more stable than the tans isomer, and the cis isomer yielded four fecal ester metabolites which resulted from hydroxylation at the 2'- and 4'-positions of the phenoxy group, at the trans- methyl group, and at both of the two latter sites. The ester-cleaved metabolites were extensively excreted into the urine, whereas the metablites retaining ester linkage were found only in the feces. There were no significant differences in metabolism between the (IRS)-isomers and (IR)-isomers. When White Leghorn hens were treated orally three consecutive days with one of four (14)C-trans- and cis-permethrin isomers labelled in the alcohol or acid at 10 mg/kg body weight, they showed no signs of poisoning. More than 87% of the radiocarbon from the four labelled perparations was found in the excreta 9 days after the initial dose, 0.7-4.7% of the dose was exhaled as (14)CO2, and 0.12-0.47% and 0.06-0.66% of the radiocarbon was recovered in egg yolk and fat (subcutaneous and visceral fat), respectively. Both the cis isomers labelled in the alcohol and acid moieties showed recoveries 3 to >10 times higher in the fat and egg yolk than those shown by the corresponding trans isomers. The excreta (0-72 hr) contained 1.7 times more cis-permethrin than trans-permethrin. Hydroxylated ester metabolites of trans-permethrin were not excreted, but four monohydroxy and dihydroxy esters (i.e. trans-OH-permethrin, 4'-OH-permethrin, 4'-OH, trans-OH-permethrin and trans-OH-permethrin sulfate) of cis-permethrin were found. Metabolites from the acid moieties of both isomers were the Cl2CA isomers in free, glucuronide, and taurine conjugate forms, trans-OH-Cl2CA, cis-OH-Cl2CA, cis-OH-Cl2CA lactone, and cis-OH-Cl2CA sulfate. trans-OH-Cl2CA was obtained from the cis isomer to larger extents than from the trans isomer, whereas the amounts of cis-OH-Cl2-CA were larger with the trans isomer than with the cis isomer. The metabolites from the alcohol moiety included PBalc, PBacid, their 4'-hydroxy-derivatives and the corresponding sulfate the glucuronide of PBalc, and a variety of unidentified conjugates of 4'-OH-PBalc and 4'-OH-PBacid. The taurine conjugate of PBacid was not detected. The metabolites produced in largest amounts were the unidentified conjugates of 4'-OH-PBalc (6-13% of the dose) and 4'-OH-PBacid (2-11%). The yolk of eggs 5 and 6 days after initial dosing contained 4.4 times cis-perethrin than trans-permethrin in unchanged form and the same ester metabolites of cis-permethrin as those found in the excreta. Other metabolites in the yolk were generally the same as those in the excreta. Overall, cis-permethrin appeared at higher levels than trans-permethrin in the egg yolk, fatty tissues, and excreta. Radiocarbon from cis-permethrin preparations also persisted longer in the blood than that from trans-permethrin preparations. It probably resulted from more rapid ester cleavage of the trans isomer than the cis isomer, based on the relative amounts of hydrolysis products form the two isomers in hen excreta. Two human volunteers, who consumed about 2 and 4 mg of permethrin (25:75), respectively, excreted 18-37% and 32-39% of the administered dose, detected as the metabolite Cl2CA, after acid hydrolysis of their urine collected over 24 hr. The permethrin metabolites in goats were formed through cleavage of the ester linkage, hydroxylation at the cis- or trans-methyl of the geminal dimethyl group, and hydroxylation at the 4'-position of the phenoxybenzyl moiety. Some of these metabolic products were further oxidized and/or conjugated with glycine, glutamic acid and glucuronic acid. The major compounds found in feces after dosing with cis-permethrin were unmetabolized parent compound 4'-OH-permethrin, trans-OH-permethrin, PBalc, cis-OH-cis-Cl2CA-lactone and eight unidentified ester metabolites. The feces of goats treated with the trans isomer contained large amounts of the parent compound (41-79% of the fecal (14)C and of PBalc (8-25%) and cis-OH-trans-Cl2CA-lactone. Also, three unidentified ester metabolites were found (8-23%). On the other hand, major urinary metabolites from the alcohol moiety of both isomers were PBacid-glycine (7-9% of the urinary (14)C) and r'-OH-PBacid-glycine (4-12%). PBalc, PBacid, 4'-OH-PBalc, 4'-OH-PBacid, PBacid-glutamic acid and 4'-OH-PBacid-glutamic acid were also identified as minor metabolites. The urine of goats treated with both isomers contained as major components, Cl2CA in the free form (2-4% of the urinary (14)C) and as a glucuronide (27-71%). Cl2CA-glucuronide was obtained to a larger extent with the trans isomer than with the cis isomer. Other major metabolites of the cis isomer were cis-OH-Cl2CA (33) (9-11%) and cis-OH-cis-Cl2CA-lactone (11-16%). trans-OH-Cl2CA was detected as a minor metabolite of both isomers. The milk of goats contained the parent compounds, PBacid-glycine, and 4'-OH-PBacide-glycine. On administration of the cis isomer, a large amount of the parent compund was excreted in the milk than in the case of the trans isomer. Comparatively, when the trans isomer was administered, PBacid-glycine was detected in the milk to a larger extent than with the cis isomer. Most of the radioactivity in the fat was attributed to the parent compound or ester metabolites such as trans-OH-permethrin and trans-OH-permethrin conjugate. For more Metabolism/Metabolites (Complete) data for PERMETHRIN (25 total), please visit the HSDB record page. The proposed metabolic pathway for cis- and trans-permethrin are as follows. The five principle sites of metabolic attack in both permethrin isomers is ester cleavage, oxidation at the trans- and cis-methyl of the geminal dimethyl group of the acid moiety, and oxidation at 2'- and 4'- position of the phenoxy group. Conjugation of the resultant carboxylic acids, alcohols, and phenols with glucuronic acid, glycine, and sulfuric acid occurs to varying extent. cis-Permethrin is more stable then trans-permethrin, and the cis isomer yields four faecally excreted ester metabolites that results from hydroxylation at the 2'- or 4'-position of the phenoxy group or at the trans- or cis methyl group on the cyclopropane ring. The estercleaved metabolites are extensively excreted into the urine whereas the metabolites retaining an ester bond are found only in the feces. The major metabolite from the acid moiety of both isomers was Cl2CA in free (1-8%) and glucuronide (14-42%) forms. Other significant metabolites are trans-OH-Cl2CA (1-5%) and cis-OH-Cl2CA in the free (3-5%), lactone (0-4%) and glucuronide (1-2%) forms. On the other hand, the alcohol moiety released after cleavage of the ester bond of both isomers is converted mainly to the sulfate of 3-(4'-hydroxyphenoxy)benzoic acid (4'-OH-PBacid) (29-43% of the dose) and PBacid in the free (1-10%) and glucuronide (7-15%) forms. Other significant metabolites of the alcohol moiety are PBalc, PBacid-glycine and the sulfate of 3-(2'-hydroxyphenoxy) benzoic acid (2'-OH-PBacid). A study by Nakamura et al. proposed that permethrin was hydrolyzed by CES (carboxylesterase), then PBAlc formed was oxidized to PBAld, and further, PBAld was oxidized to PBAcid by the P450 system in rat liver microsomes. (A559, A256) Route of Elimination: Permethrin is rapidly metabolized by ester hydrolysis to inactive metabolites which are excreted primarily in the urine. Biological Half-Life The toxicokinetics of permethrin after single 460 mg/kg oral and 46 mg/kg intravenous doses were studied in male Sprague-Dawley rats. Serial blood samples after oral and intravenous dosage, and brain, medulla oblongata, sciatic nerve, and liver samples after oral administration were collected. Plasma, hypothalamus, cerebellum, frontal cortex, caudate putamen, hippocampus, medulla oblongata, sciatic nerve, and liver concentrations of permethrin and its metabolites, m-phenoxybenzyl alcohol and m-phenoxybenzoic acid, were determined by a high-performance liquid chromatographic assay. The permethrin plasma profile could be adequately described by a two-compartment open model. For permethrin, the elimination half-life (t1/2 beta) and the mean residence time from plasma were 8.67 and 11.19 hr after i.v. and 12.37 and 17.77 hr after /peroal/ administration. The total plasma clearance was not influenced by dose concentration or route and reached a value of 0.058 liter/hr. After the single oral dose, permethrin was absorbed slowly with a Tmax of 3.52 hr. The maximum plasma concentration was 49.46 micrograms/ml. The oral bioavailability of permethrin was found to be 60.69%. The plasma concentration-time data for permethrin metabolites as well as the tissue concentration-time data for permethrin and its metabolites after an oral dose of permethrin were found to fit a one-compartment open model. The elimination half-life (t1/2el) of permethrin was greater for the hippocampus, medulla oblongata, frontal cortex, and sciatic nerve (23.10, 22.36, 13.86, and 16.27 hr, respectively) than for plasma (t1/2 beta, 12.37 hr). The maximum amounts of permethrin in cerebellum, hippocampus, caudate putamen, frontal cortex, hypothalamus, and sciatic nerve were about 1.5, 2, 2, 2.7, 4.8, and 7.5 times higher than in plasma, respectively, indicating an accumulation of pyrethroid by nervous tissue itself. Nervous tissue accumulation of permethrin was also reflected by the area under the concentration curve ratios of tissue/plasma (1.16, 3.71, 1.57, 4.27, 3.48, and 8.77, respectively). The metabolites of permethrin, m-phenoxy-benzyl alcohol and m-phenoxybenzoic acid, were detected in plasma and in all selected tissues for 48 hr after dosing, suggesting that a combination of metabolism by the tissues and diffusion into it from the blood may be present. In studies, the half-life of (+)-trans- and (+)-cis-permethrin applied to the leaf surface of bean plants was 7 and 9 days, respectively. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Pyrethroids exert their effect by prolonging the open phase of the sodium channel gates when a nerve cell is excited. They appear to bind to the membrane lipid phase in the immediate vicinity of the sodium channel, thus modifying the channel kinetics. This blocks the closing of the sodium gates in the nerves, and thus prolongs the return of the membrane potential to its resting state. The repetitive (sensory, motor) neuronal discharge and a prolonged negative afterpotential produces effects quite similar to those produced by DDT, leading to hyperactivity of the nervous system which can result in paralysis and/or death. Other mechanisms of action of pyrethroids include antagonism of gamma-aminobutyric acid (GABA)-mediated inhibition, modulation of nicotinic cholinergic transmission, enhancement of noradrenaline release, and actions on calcium ions. (T18, L857) Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because less than 2% is absorbed after topical application, rapid metabolism to inactive metabolites and safe application directly on infants' skin, topical permethrin products are acceptable in nursing mothers. Extensive exposure, such as from agricultural use or malaria control might have long-term health concerns because residues can be found in breastmilk. Only water-miscible cream, gel or liquid products should be applied to the breast because ointments may expose the infant to high levels of mineral paraffins via licking. ◉ Effects in Breastfed Infants In a telephone follow-up study, 5 mothers who used permethrin during breastfeeding reported no adverse reactions in their breastfed infants. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Toxicity Data LC50 (rat) = 485 mg/m3 Oral, rat LD50: 430 - 4000 mg/kg Skin, rabbit LD50: 2000 mg/kg LD50: 3 801 mg/kg (Oral, Rat) (L857) Interactions The effects of pyrethroids were studied on phosphoinositide breakdown in guinea pig synaptoneurosomes. Similar to other agents that activate voltage-dependent sodium channels, type I and type II pyrethroids stimulated phosphoinositide breakdown. Type II pyrethroids, like deltamethrin and fenvalerate, were more potent and, at least for deltamethrin, more efficacious than type I pyrethroids, like allethrin, resmethrin and permethrin. The effects of type II pyrethroids could be partially inhibited by the sodium channel blocker tetrodotoxin. The effects of allethrin and resmethrin were not affected by 5 microM tetrodotoxin. Stimulation of phosphoinositide breakdown by fenvalerate was additive to the stimulation elicited by the receptor agonists carbamylcholine and norepinephrine, but not to the stimulation elicited by sodium channel agents (batrachotoxin, scorpion venom and pumiliotoxin B). Stimulation by allethrin was not additive to the stimulation elicited either by receptor agonists or sodium channel agents. A submaximal concentration of allethrin, a type I pyrethroid, did not greatly affect the dose-dependent stimulation elicited by a type II pyrethroid, deltamethrin, while a higher concentration of allethrin prevented further stimulation by type II pyrethroids. A local anesthetic, dibucaine, which inhibits sodium channel activation, inhibited phosphoinositide breakdown induced by type II, but not by type I pyrethroids, except at higher concentrations. Thus, type II pyrethroids appear to stimulate phosphoinositide breakdown in synaptoneurosomes in a manner analogous to other sodium channel agents, while type I pyrethroids elicit phosphoinositide breakdown by a different mechanism, probably not involving sodium channels. /Pyrethroid/ detoxification ... important in flies, may be delayed by the addition of synergists ... organophosphates or carbamates ... to guarantee a lethal effect. ... /Pyrethroid/ Piperonyl butoxide potentiates /insecticidal activity/ of pyrethrins by inhibiting the hydrolytic enzymes responsible for pyrethrins' metabolism in arthropods. When piperonyl butoxide is combined with pyrethrins, the insecticidal activity of the latter drug is increased 2-12 times /Pyrethrins/ At dietary level of 1000 ppm pyrethrins and 10000 ppm piperonyl butoxide ... /enlargement, margination, and cytoplasmic inclusions in liver cells of rats/ were well developed in only 8 days, but ... were not maximal. Changes were proportional to dosage and similar to those produced by DDT. Effects of the 2 ... were additive. /Pyrethrins/ /Nuclear magnetic resonance/ (NMR) combined with pattern recognition was recently introduced as a new technique for rapid xenobiotic toxicity evaluation. In this article, metabolic changes in the biofluid of rats after 90-day oral treatment with propoxur, permethrin and a combination of these two pesticides were investigated. Propoxur dosing induced increased urinary taurine, creatinine and glucose, whereas urinary lactate and acetate were increased in the highest permethrin dose group. Urinary acetate, alanine, lactate and trimethylamine levels were increased in the mixture group, accompanied by decreased urinary tricarboxylic acid cycle intermediates. In addition, the highest dose of the mixture displayed raised 3-D-hydroxybutyrate, acetate and lactate levels in the serum sample. Chronic exposure to a combination of propoxur and permethrin may induce hepatotoxicity and nephrotoxicity. An increase in acetate, alanine and formate in the urine could be a potentially sensitive biomarker of the chronic, combined effects of permethrin and propoxur. Non-Human Toxicity Values LD50 Rat oral 1,500 mg/kg LD50 Rat oral 600 mg/kg LD50 Rat oral 1,300 mg/kg LD50 Rat oral 430-4000 mg/kg /cis:trans-isomer ratio of 40:60/ For more Non-Human Toxicity Values (Complete) data for PERMETHRIN (30 total), please visit the HSDB record page. |
| 其他信息 |
Therapeutic Uses
MEDICATION (VET) Crusted (Norwegian) scabies, a rare variant of ordinary scabies, is a highly contagious infection in which the skin is infested with thousands to millions of mites. The infection is frequently overlooked because of its atypical presentations. Patients with cognitive deficiency or an immunodeficiency disorder (including immunosuppressive therapy) are predisposed to developing crusted scabies. The infection often presents as generalized dermatitis with crusted hyperkeratosis on the palms and soles. Diagnosis is made by examining skin scrapings from the crusted lesions. Lindane is the scabicide most widely used in the treatment of crusted scabies. Eradication frequently requires repeated applications, and care must be taken to avoid lindane toxicity. Permethrin cream is as efficacious as lindane in the treatment of ordinary scabies. Because of its wider margin of safety, permethrin may become the preferred treatment for crusted scabies. Permethrin is a synthetic pyrethroid that has low mammalian toxicity and an insecticidal effectiveness higher that of the natural pyrethrins. Because of its high ovicidal activity and persistence on hair, a properly applied 1% cream rinse preparation eliminates head lice infestation after a single application. Fewer than 1% of patients have required retreatment after seven days. Permethrin exerts ovicidal effects against lice, and some activity may result from residues that remain on the hair for 2 weeks or longer that kill nymphs as they emerge from eggs.In one in vitro study using live lice and viable nits obtained from healthy lice-infested children in Panama, exposure to permethrin 1% cream rinse killed 30% of the lice within 5 minutes, 53% within 10 minutes, and 100% within 1 hour; 89% of the nits were killed within 10 minutes. However, when permethrin 1% was diluted 10:1 in water to approximate the dilution that occurs when the drug is applied to wet hair, 11% of the lice were killed within 5 minutes, 44% within 10 minutes, and 94% within 1 hour; 81% of the nits were killed within 10 minutes when exposed to the diluted solution. For more Therapeutic Uses (Complete) data for PERMETHRIN (19 total), please visit the HSDB record page. Drug Warnings The safety and effectiveness of permethrin in children less than 2 years of age have not been established. Patients who cannot tolerate chrysanthemums, pyrethrins, and other synthetic pyrethroids may not tolerate permethrin. Pharmacodynamics Permethrin, a pyrethroid, is active against a broad range of pests including lice, ticks, fleas, mites, and other arthropods. |
| 分子式 |
C21H20CL2O3
|
|---|---|
| 分子量 |
391.29
|
| 精确质量 |
390.078
|
| CAS号 |
52645-53-1
|
| 相关CAS号 |
Permethrin-d5;1794760-19-2;Permethrin-d9;trans-Permethrin;61949-77-7
|
| PubChem CID |
40326
|
| 外观&性状 |
Colorless crystals to a viscous liquid; Color, white to pale yellow
Pure permethrin cis-isomer forms colorless crystals at room temperature but a mixture of cis and trans isomers normally occurs as a liquid, with its appearance depending on the ratio of isomers. Pure permethrin (40:60) is a colorless, viscous liquid, whereas the technical compound is a yellow to yellow-brown viscous liquid. |
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
465.9±45.0 °C at 760 mmHg
|
| 熔点 |
34-35°C
|
| 闪点 |
159.4±27.7 °C
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
| 折射率 |
1.616
|
| LogP |
7.15
|
| tPSA |
35.53
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
26
|
| 分子复杂度/Complexity |
521
|
| 定义原子立体中心数目 |
0
|
| SMILES |
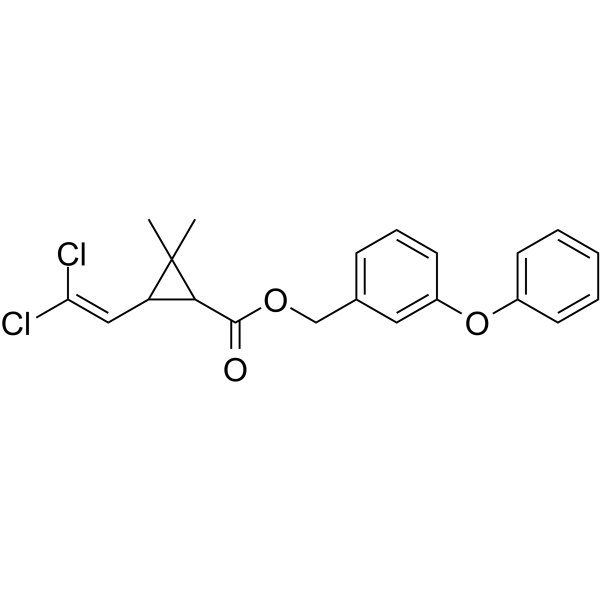
Cl/C(=C(/[H])\C1([H])C([H])(C(=O)OC([H])([H])C2C([H])=C([H])C([H])=C(C=2[H])OC2C([H])=C([H])C([H])=C([H])C=2[H])C1(C([H])([H])[H])C([H])([H])[H])/Cl
|
| InChi Key |
RLLPVAHGXHCWKJ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C21H20Cl2O3/c1-21(2)17(12-18(22)23)19(21)20(24)25-13-14-7-6-10-16(11-14)26-15-8-4-3-5-9-15/h3-12,17,19H,13H2,1-2H3
|
| 化学名 |
(3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate
|
| 别名 |
1RS,cis-Permethrin; Transpermethrin; Permethrin
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~50 mg/mL (~127.78 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.39 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (6.39 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.39 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5556 mL | 12.7782 mL | 25.5565 mL | |
| 5 mM | 0.5111 mL | 2.5556 mL | 5.1113 mL | |
| 10 mM | 0.2556 mL | 1.2778 mL | 2.5556 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Comparison of Oral Ivermectin and Permethrin 5% Lotion in Treatment of Pediculosis Capitis
CTID: NCT05643820
Phase: Phase 1 Status: Completed
Date: 2022-12-09