| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
FGFR1 (IC50 = 10 nM); cFMS (IC50 = 20 nM); FLT3 (IC50 = 160 nM); KDR (IC50 = 350 nM); LCK (IC50 = 860 nM); FLT1 (IC50 = 880 nM); NTRK3 (IC50 = 890 nM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:在M-NFS-60、Bac1.2F5和M-07e细胞中,Pexidartinib抑制CSF1依赖性增殖,IC50分别为0.44 μM、0.22 μM和0.1 μM。激酶检测:Pexidartinib (PLX-3397) 是一种有效、选择性和 ATP 竞争性 CSF1R (cFMS) 和 c-Kit 抑制剂,对 c-Kit 和 CSF1R 的选择性比其他相关激酶(例如 FLT3)高 10 至 100 倍、KDR (VEGFR2)、LCK、FLT1 (VEGFR1) 和 NTRK3 (TRKC),IC50 分别为 160、350、860、880 和 890 nM。
CSF1R活性在体外促进T细胞淋巴瘤生长 [5] 在明确T细胞淋巴瘤(TCL)中CSF1R的表达与激活后,我们采用功能缺失策略,通过分子与药理学互补方法探究其潜在致癌作用。首先使用临床可用且合理设计的CSF1R选择性酪氨酸激酶抑制剂(培西达替尼,PLX3397)(25,49)。为验证培西达替尼对CSF1R的抑制效果,用培西达替尼处理具有CSF1R自分泌激活的TCL细胞。结果显示培西达替尼治疗后CSF1R磷酸化显著降低(图2A,补充图4A)。重要的是,培西达替尼对部分TCL细胞中表达的致癌激酶NPM-ALK的磷酸化水平无影响(补充图4B)。此外,培西达替尼暴露导致细胞增殖呈剂量依赖性下降(图2B及补充图4D-E),但在不表达CSF1R的TCL细胞中未观察到此效应,证实了该FDA批准药物的相对选择性(补充图4C)。与此一致,培西达替尼治疗增加TCL细胞凋亡,表现为磷脂酰丝氨酸外翻(图2C-E)、PARP裂解及Caspase 3裂解(图2F与补充图4F)。为深入探究CSF1R在T细胞淋巴瘤生长中的作用并排除TKI脱靶效应可能,成功在T细胞淋巴瘤衍生细胞系中建立多西环素诱导的CSF1R靶向shRNA稳定表达体系。 CSF1R激活与不同信号通路磷酸化相关 [5] CSF1R的生理性结合与激活引发下游磷酸化依赖性信号传导,调控髓系细胞存活与分化(33,34)。然而,CSF1R激活在多数非髓系细胞中调控的下游信号尚不明确。为表征CSF1R激活的信号通路,在T细胞淋巴瘤系中用培西达替尼抑制CSF1R活性后,采用无偏倚磷酸化蛋白质组学分析。筛选选用Karpas 299 T细胞淋巴瘤系,因其兼具CSF1配体分泌与强表面CSF1R表达。该筛选共鉴定1936个独立磷酸酪氨酸肽段(对应1123种蛋白质),同时识别8045个独立磷酸丝氨酸/磷酸苏氨酸肽段组合(对应3136种蛋白质)(补充表1-2)。培西达替尼处理细胞中CSF1R自身磷酸化肽段显著减少,进一步验证了该方法(表1,补充图5A及补充表3)。对三个技术重复进行层次聚类分析,显示培西达替尼处理细胞形成独立聚类(图4A与补充图5B),共涵盖551种磷酸化肽段显著修饰的独特蛋白。其中451个为丝氨酸残基修饰,122个为酪氨酸残基修饰,113个为苏氨酸残基修饰(补充表3)。通过京都基因与基因组百科全书(KEGG在线软件)对CSF1R抑制后的信号通路分析显示,这些变化与PI3K/AKT调控信号通路的差异性磷酸化一致(补充表1,图4B)。此外,还鉴定到参与细胞过程(包括代谢、细胞周期进程和肌动蛋白-细胞骨架动力学)的蛋白质修饰(图4B,补充表4及补充图5C)。为深入探索CSF1R信号下游激活的通路,在培西达替尼抑制CSF1R后进行无偏倚基因表达谱芯片分析。经培西达替尼抑制CSF1R后,217个基因表达显著改变(n=3, p<0.01;图4C)。重要的是,CSF1R抑制与细胞因子(JAK/STAT)信号相关基因表达变化相关(图4D)。类似地,磷酸化蛋白质组学筛选显示JAK/STAT信号通路相关蛋白(包括STAT1、STAT3、STAT5和SOS2)存在差异性磷酸化(补充表2)。因筛选所用T细胞系携带致癌性NPM-ALK融合,故纳入ALK抑制剂克唑替尼处理细胞作为对照。与ALK抑制相比,培西达替尼处理细胞中46%的基因表达变化(n=154个基因)具有特异性(图4E),63个基因的表达受培西达替尼或克唑替尼共同调控(图4E)。 |
| 体内研究 (In Vivo) |
在 MMTV-PyMT 小鼠中,Pexidartinib(40 mg/kg,口服)通过 CD45+CD11b+Ly6C−Ly6G−F4/80+ 显着抑制稳态和 PTX 诱导的肿瘤浸润。 Pexidartinib/PTX 治疗还导致乳腺肿瘤内 CD31+ 血管密度显着降低,同时诱导细胞凋亡和坏死。在携带 GL261 肿瘤的 C57 小鼠中,Pexidartinib (po) 抑制胶质母细胞瘤侵袭。在 cmo 小鼠中,PLX3397 通过减少尾部和爪子的侵蚀性骨病变以及循环 MIP-1α 的水平,显着减轻自身炎症性疾病。在患有 B16F10 黑色素瘤的小鼠中,Pexidartinib(45 mg/kg,口服)可增强 CD8 介导的黑色素瘤免疫治疗。[1]
PLX3397治疗显著降低了喂食食物和高脂肪饮食的小鼠脂肪组织中的巨噬细胞数量,而不影响总髓细胞水平。尽管如此,PLX3397并没有显著改变葡萄糖稳态,也没有影响高脂肪饮食诱导的内脏脂肪细胞因子表达(Il-6和Tnfa)的增加,对应激激酶JNK和ERK的磷酸化以及巨噬细胞极化的影响有限。 化疗诱导的TAM募集的阻断 [1] 为探究肿瘤浸润性TAM是否调控乳腺上皮细胞(MEC)对细胞毒疗法的敏感性,我们通过免疫学和药理学手段在体内阻断TAM浸润(补充图S4),并评估治疗小鼠肿瘤的髓系细胞浸润情况(补充图S5)。荷原位乳腺肿瘤的小鼠接受中和性单克隆抗体(mAb)CSF1(克隆5S1)或CD11b(克隆M1/70)治疗,或采用对CSF1/cKIT受体酪氨酸激酶具有高效(nM级)选择性的ATP竞争性抑制剂(培西达替尼),实施单药或与紫杉醇(PTX)联用治疗。CD11b是表达于粒细胞、巨噬细胞、单核细胞、树突状细胞(DC)及自然杀伤细胞的整合素细胞黏附分子,部分调控细胞向组织和肿瘤实质的跨内皮迁移。PLX3397对cKIT和CSF1R的选择性较其他相关激酶(如KDR)高10-100倍(见补充图S6A与方法;参考文献20)。 对浸润乳腺肿瘤的主要髓系亚型进行荧光激活细胞分选分析显示:无论单药或联用PTX,经αCSF1单抗或培西达替尼治疗后,CD45+CD11b+Ly6C−Ly6G−F4/80+ TAM的募集均显著减少,但对CD45+CD11b+LY6Ghigh 未成熟髓系细胞(iMC)或CD45+CD11blow/−Ly6C−CD22−Ly6G−CD11chighMHCIIhigh DC的浸润无影响(图3A、B;补充图S5A、B)。αCD11b单抗治疗则同时降低TAM和iMC浸润(图3A)。分析经αCSF1或PLX3397治疗后残存于乳腺肿瘤组织的TAM成熟分化状态,发现CD11b、CD11c、F4/80、CD45及MHCII表达均无显著变化(补充图S5B)。但乳腺肿瘤切片检查显示仍存在一群血管周非CSF1依赖的F4/80+ TAM(图3C)。TAM募集的阻断是CSF1/CSF1R抑制的直接效应:体外CSF1R抑制有效阻断了CD11b+单核细胞对对照组或PTX处理的pMEC条件培养基的趋化反应,而对CD3+ T淋巴细胞趋化无影响(图3D;补充图S3G)。该结果在体内得到印证:晚期MMTV-PyMT小鼠经PLX3397治疗显著抑制了CD45+CD11b+Ly6C−Ly6G−F4/80+ TAM的基础状态及PTX诱导的肿瘤浸润(图3E;补充图S5),且未改变TAM成熟/分化状态(补充图S6B)。 随后,我们以αCSF1、αCD11b或培西达替尼(对比对照组)治疗80日龄MMTV-PyMT小鼠或荷同基因原位PyMT来源肿瘤(约1.0 cm)的小鼠5天,继以4周期PTX(10 mg/kg,静脉注射;补充图S4)。研究终点(原发瘤达2.0 cm或小鼠100日龄)时,αCSF1/PTX、αCD11b/PTX或PLX3397/PTX联合治疗组小鼠的原发瘤负荷较单药治疗组显著降低(图4A、B;补充图S7A)。在接受PLX3397/卡铂(CBDCA)联合治疗的同基因荷PyMT来源乳腺肿瘤小鼠中也观察到类似结果(图4B)。 MMTV-PyMT小鼠乳腺肿瘤的进展遵循明确的癌症发展阶段,与女性乳腺癌进程相似,包括旺炽型导管增生组织、伴早期间质浸润的导管原位癌及低分化浸润性导管癌(15, 21)。基于此分期标准,我们发现:与同龄单用PTX或培西达替尼治疗的小鼠相比,接受培西达替尼/PTX联合治疗的小鼠乳腺肿瘤晚期癌变进展减少(图4C;补充图S7B)。此外,PLX3397/PTX治疗组小鼠中出现的晚期癌灶存在大面积坏死(补充图S7C),其特征为凋亡细胞增多(通过cleaved caspase 3阳性检测;图4D),而上皮增殖无伴随改变(补充图S7D)。 血管密度降低伴随化疗敏感性提升 [1] 已知TAM为发育中乳腺肿瘤提供VEGF,从而调控组织血管生成程序(22–24)。MMTV-PyMT小鼠对顺铂(CDDP)的化疗敏感性部分受髓系来源VEGF调控(25);因此,我们探究TAM清除是否改变PTX治疗的MMTV-PyMT小鼠的VEGF表达和/或CD31+血管密度。虽然培西达替尼显著降低总VEGF mRNA表达(图4E),但70%的降幅与血管密度变化无关(图4F)。相比之下,PLX3397/PTX联合治疗导致乳腺肿瘤内CD31+血管密度显著降低,且与凋亡及坏死诱导同步(图4F)。 CSF1信号阻断增强化疗诱导的抗肿瘤免疫及CTL浸润 [1] 因人类乳腺癌组织分析显示基质TAM密度与CD8+ T细胞浸润呈负相关(补充表S1),我们推测清除TAM将促进CD8+ CTL浸润,从而营造抗肿瘤免疫微环境。通过流式细胞术或免疫组化(IHC)分析αCSF1/PTX或培西达替尼/PTX治疗小鼠的肿瘤浸润T淋巴细胞,发现乳腺肿瘤中CD4+和CD8+ T细胞显著增加(图5A、B;补充图S8A)。与此一致,PLX3397/PTX治疗的MMTV-PyMT小鼠乳腺组织细胞因子mRNA表达显示:细胞毒性效应分子(如IFN-γ、颗粒酶A、颗粒酶B、穿孔素-1)及1型DC效应分子(IL12p35、IFN-α)的mRNA表达升高(图5C)。相反,免疫抑制分子精氨酸酶-1的表达因PLX3397/PTX治疗下降(图5C)。此免疫微环境重编程伴随CD45+CD11blow/−CD19−Ly6G−Ly6ClowCD11chighMHCIIhigh DC的肿瘤浸润增加(图5D),表明PLX3397/PTX联合治疗通过表达高水平细胞毒性效应分子的T淋巴细胞激发抗肿瘤免疫应答。 巨噬细胞清除以CD8∙ CTL依赖方式增强化疗反应 [1] 为探究培西达替尼/PTX治疗小鼠乳腺肿瘤化疗敏感性增强是否依赖CD8+ T细胞应答,我们在接受PTX、PLX3397或两者联用的晚期MMTV-PyMT小鼠中清除CD8+ T细胞。研究证实:PLX3397/PTX联合治疗提升的化疗敏感性及疗效改善确为CD8+ T细胞依赖性反应(图6A、B;补充图S8B)。我们发现CD8清除还导致接受PLX3397/PTX联合治疗的小鼠肿瘤分级升高且cleaved caspase-3阳性细胞减少(图6C、D)。综上,这些数据表明CSF1R信号阻断引发的细胞毒性反应增强具有CD8+ T细胞依赖性。 巨噬细胞清除联合化疗以CD8依赖方式阻断转移 [1] 乳腺癌患者长期生存常因原发瘤手术切除后播散性转移受限。人类乳腺癌白细胞谱分析显示总生存期(OS)及转移扩散受肿瘤浸润T淋巴细胞和巨噬细胞谱系调控。在MMTV-PyMT小鼠中,尽管单一CSF1R信号阻断或PTX治疗均未抑制肺转移发生,但接受培西达替尼/PTX联合治疗的小鼠肺转移减少>85%,且部分依赖CD8+ T细胞(图6E)。 培西达替尼治疗新生小鼠减少小胶质细胞及BrdU阳性增殖细胞 [2] PLX3397(一种集落刺激因子1受体(CSF1R)抑制剂)常用于清除小胶质细胞。本研究中,新生小鼠经腹腔注射PLX3397(图3A)。从出生后第0天(P0)至P7每日两次给药显著减少小胶质细胞数量(p=0.015,图3B,C)。PLX3397还降低视网膜距视神经500 μm处BrdU阳性增殖细胞数量(p=0.021,图3D,E)。为验证增殖细胞为视网膜前体细胞,我们共标BrdU与视网膜前体细胞标志物Pax6、Chx10(亦称视觉系统同源框2(Vsx2))。PLX3397显著减少BrdU/Chx10双阳性细胞,且呈减少BrdU/Pax6双阳性细胞趋势(p=0.038,图3F,G),但未改变cleaved caspase-3阳性细胞数量(图3H)。 培西达替尼/PLX3397治疗显著降低普通和高脂饮食小鼠脂肪组织巨噬细胞数量,但不影响总髓系细胞水平。尽管如此,PLX3397未明显改变葡萄糖稳态,未影响高脂饮食诱导的内脏脂肪细胞因子(Il-6和Tnfa)表达升高,且对应激激酶JNK和ERK磷酸化及巨噬细胞极化影响有限。 结论:高脂饮食诱导的脂肪组织巨噬细胞浸润可能并非机体葡萄糖稳态受损的触发因素,抗CSF1疗法或不适于治疗胰岛素抵抗 [4]。 激活CSF1R促进PTCL体内生长[5] 为了进一步研究这些发现的治疗相关性,我们通过 培西达替尼抑制CSF1R来评估t细胞淋巴瘤异种移植物的生长。小鼠CSF1不结合人CSF1R(48,59);因此,我们在NSG小鼠产生的Karpas 299异种移植物中评估了自分泌依赖性CSF1R的激活。荷瘤小鼠用含假或培西达替尼的鼠粮治疗,没有发现与治疗相关的毒性。培西达替尼治疗小鼠肿瘤生长减少约50% (n=24, p<0.05;图6A和B),并且在培西达替尼处理的肿瘤蛋白提取物中观察到细胞凋亡增加(图6C)。使用这些异种移植物的蛋白质提取物检测CSF1R (Y699)和p70S6K (T389)的磷酸化作为药效学生物标志物,在培西达替尼处理的小鼠中观察到磷酸化显著降低(图6D)。在类似设计的实验中,使用supp - m2细胞(需要外源性CSF1),并在转基因表达人CSF1(48)或非CSF1表达对照的免疫缺陷小鼠中产生异种移植物。与对照小鼠相比,CSF1生成小鼠的肿瘤体积增加了约3倍(n=32, p<0.001;图6E和F)。重要的是,培西达替尼治疗的CSF1转基因小鼠的肿瘤生长受到抑制(n=15, p<0.001;图6E和F)。然而,在培西达替尼治疗的对照组小鼠中,肿瘤生长没有明显变化(图6E和F)。总的来说,这些发现表明,CSF1R的激活,无论是自分泌依赖还是旁分泌依赖,都能促进t细胞淋巴瘤的生长,并进一步支持CSF1R作为这些淋巴瘤的合理治疗靶点(补充图7)。 |
| 酶活实验 |
Pexidartinib (PLX3397)的生化选择性和效力:[1]
Pexidartinib(PLX3397)选择性抑制c-Fms和c-Kit受体酪氨酸激酶,生化IC50值分别为0.02µM和0.01µM(图S6A)Pexidartinib(PLX3397)通过使用基于支架和X射线结构的发现方法被鉴定为一种有效的CSF-1R和c-KIT激酶抑制剂。在226种不同激酶的全面筛选中,包括所有蛋白激酶亚家族和几种脂质激酶的代表,0.03µM和1.0µM的Pexidartinib (PLX3397)仅显著抑制了其他五种激酶Pexidartinib(PLX3397)是基于抑制小鼠粒细胞白血病细胞系M-NFS-60的CSF1依赖性增殖而选择的,IC50为0.44µM,小鼠巨噬细胞系Bac1.2F5的IC50为0.22µM。依赖添加SCF生长的人急性巨核细胞白血病细胞系M-07e被Pexidartinib (PLX3397)抑制,IC50为0.1µM。这些亚微摩尔效力证实,培昔达替尼(PLX3397)可以进入细胞并抑制Fms驱动的细胞生长。[1] Pexidartinib (PLX-3397) 是一种 ATP 竞争性、强效、选择性的 CSF1R (cFMS) 和 c-Kit 抑制剂,与其他相关激酶、FLT3、KDR (VEGFR2)、LCK、FLT1 相比,对 c-Kit 和 CSF1R 具有选择性(VEGFR1) 和 NTRK3 (TRKC),IC50 值分别为 160、350、860、880 和 890 nM。 |
| 细胞实验 |
CSF1R活性促进T细胞淋巴瘤体外生长[1]
在确定CSF1R在TCL中的表达和激活后,我们采用了功能丧失策略,通过互补的分子和药理学方法来解决其在这些TCL中潜在的致癌作用。我们首先使用了一种临床上可用且设计合理的酪氨酸激酶抑制剂,该抑制剂对CSF1R具有选择性(Pexidartinib,PLX3397)。为了证实pexidartinib处理对CSF1R的抑制作用,用pexidarinib处理具有CSF1R自分泌激活的TCL细胞。用pexidartinib治疗后,观察到CSF1R磷酸化显著降低(图2A,补充图4A)。重要的是,pexidartinib对在部分评估的TCL细胞中表达的致癌激酶NPM-ALK的磷酸化水平没有显示出任何影响(补充图4B)。此外,暴露于pexidartinib后,观察到增殖呈剂量依赖性下降(图2B和补充图4D-E),但在不表达CSF1R的TCL细胞中没有观察到这些影响,这支持了这种FDA批准的药物的相对选择性(补充图4C)。与这些发现一致,pexidartinib治疗与TCL细胞凋亡增加有关,如磷脂酰丝氨酸暴露(图2C-E)、PARP切割和Caspase 3切割所示。[1] 通过应用基于支架和 X 射线结构的发现方法,发现 PLX3397 是 CSF-1R 和 c-KIT 激酶的强抑制剂。 SelectScreenTM 分析服务提供了 IC50 数据。 白细胞趋化性实验:为了细胞迁移,从心脏穿刺后的FVB/n小鼠外周血中收集pbl,并将其(105个细胞/ 100µl含0.1% BSA的DMEM)播种到transwell过滤器的顶部腔(3-µm)。将过滤器放置在24孔板中,该板含有从车辆或PTX (20 nM)预处理的mmtv - pymt衍生mec分离的条件培养基。在某些条件下,上腔中加入Pexidartinib/PLX3397 (50 nM)。孵育6小时后,分离下室培养基,流式细胞术分析CD11b、CD3和7AAD。每个实验组取3份样品,重复实验2次。[1] |
| 动物实验 |
MMTV-PyMT mice
40 mg/kg/day p.o. Two murine models of mammary tumor development were used to analyze response to chemotherapy (Supplementary Fig. S3). The first model used MMTV-PyMT mice (Supplementary Fig. S3A). The 80-day-old MMTV-PyMT female littermates were randomized by initial tumor volume and fed either PLX3397 formulated in mouse chow or control chow (provided by Plexxikon Inc). Pexidartinib/PLX3397 was formulated in mouse chow so that the average dose per animal per day was 40 mg/kg. When PLX3397-treated MMTV-PyMT mice reached 85 days of age, they were then administered PTX every 5 days by i.v. injection into the retroorbital plexus. PTX was given at 10 mg/kg of the animal per injection, diluted in PBS. Tumor burden was evaluated by caliper measurement every 5 days following the start of PLX3397 treatment. Prior to tissue collection, mice were cardiac-perfused with PBS to clear peripheral blood. Mammary tumor tissue from PBS-perfused MMTV-PyMT mice was analyzed by flow cytometry and qRT-PCR 2 days after the second dose of PTX, when metastatic burden and tumor grade were determined. Primary tumor burden was determined by caliper measurements on live sedated mice. Metastatic burden was assessed by serial sectioning of formalin-fixed paraffin-embedded lung tissue whereby the entire lung was sectioned and the number of metastatic foci (>5 cells) was determined on 6 sections taken every 100 µm following H&E staining. Lungs from >10 mice/group were analyzed[1]. A Ten-week-old mice were fed a chow or high-fat diet for 10 weeks and then treated with Pexidartinib/PLX3397 via oral gavage (50 mg/kg) every second day for 3 weeks, with subsequent monitoring of glucose tolerance, insulin sensitivity and assessment of adipose tissue immune cells.PLX3397 treatment substantially reduced macrophage numbers in adipose tissue of both chow and high-fat diet fed mice without affecting total myeloid cell levels. Despite this, PLX3397 did not greatly alter glucose homeostasis, did not affect high-fat diet-induced increases in visceral fat cytokine expression (Il-6 and Tnfa) and had limited effect on the phosphorylation of the stress kinases JNK and ERK and macrophage polarization.[4] Preclinical Mouse Models and Animal Husbandry [1] Mice harboring the PyMT transgene under the control of the MMTV promoter in the FVB/n strain were used. Two murine models of mammary tumor development were used to analyze response to chemotherapy (Supplementary Fig. S3). The first model used MMTV-PyMT mice (Supplementary Fig. S3A). The 80-day-old MMTV-PyMT female littermates were randomized by initial tumor volume and fed either Pexidartinib/PLX3397 formulated in mouse chow or control chow. PLX3397 was formulated in mouse chow so that the average dose per animal per day was 40 mg/kg. When PLX3397-treated MMTV-PyMT mice reached 85 days of age, they were then administered PTX every 5 days by i.v. injection into the retroorbital plexus. PTX was given at 10 mg/kg of the animal per injection, diluted in PBS. Tumor burden was evaluated by caliper measurement every 5 days following the start of PLX3397 treatment. Prior to tissue collection, mice were cardiac-perfused with PBS to clear peripheral blood. Mammary tumor tissue from PBS-perfused MMTV-PyMT mice was analyzed by flow cytometry and qRT-PCR 2 days after the second dose of PTX, when metastatic burden and tumor grade were determined. Primary tumor burden was determined by caliper measurements on live sedated mice. Metastatic burden was assessed by serial sectioning of formalin-fixed paraffin-embedded lung tissue whereby the entire lung was sectioned and the number of metastatic foci (>5 cells) was determined on 6 sections taken every 100 µm following H&E staining. Lungs from >10 mice/group were analyzed. To assess tumor grade, the stage characterization technique classified tumor tissue into 3 levels of histologic progression by quantifying the area of transformed glands occupied by each stage. Progression follows from a “precancerous stage” characterized by premalignant hyperplasia and adenoma/mouse intestinal epithelium but with the retention of some normal ductal and acinar mammary gland morphology, to a more epithelial cell–dense “early carcinoma” with some stromal invasion, and finally to an invasive, high–mitotic index “late-stage carcinoma.” The IHC analysis was conducted on tissue sections following the end of studies on 100-day-old MMTV-PyMT mice (detailed in Supplementary Fig. S4A). Vehicle-treated mice received PBS-only injections. We also used a syngeneic orthotopic implantable tumor model (referred to as PyMT-implantable in all figures and detailed in Supplementary Fig. S4B). For this model, single-cell suspensions of tumor cell pools isolated from mammary tumors of 3 or 4 100-day-old MMTV-PyMT mice were generated following collagenase A digestion (see discussion of flow cytometry analysis earlier). A total of 1.0 million tumor cells from pools were diluted in medium and basement membrane extract and injected orthotopically into uncleared mammary fat pads (4th gland) of 10-week-old virgin FVB/n female mice. Following implantation, tumors were allowed to grow to a mean diameter of 1.0 cm before enrollment into studies. Mice were randomized into treatment groups based on tumor size and treated with Pexidartinib/PLX3397 and PTX, as described above. For some studies, CBDCA was used and administered at 10 mg/kg of mouse per injection, in a similar manner to administration of PTX (see above). For mice with implantable tumors, tumor burden was evaluated by caliper measurement every 2 to 3 days following the start of PLX3397 treatment, and mammary tissue was analyzed by flow cytometry, IHC, and qRT-PCR at the end of the study (Supplementary Fig. S3B). Immune-depleted mice were injected i.p. every 5 days with either 1.0 mg anti-CD8 immunoglobulin G (YTS169.4) or isotype control rat immunoglobulin on day 1 followed by 500 µg every 5 days. PLX3397/Pexidartinib treatment [2] PLX3397 (Pexidartinib) was treated at a dose of 0.25 and 1 mg/kg (i.p., twice daily) to neonatal mice from P0 to P7. PLX3397 250 mg/ml in 100% dimethyl sulfoxide (DMSO) was prepared as the stock solution. The stock solution was diluted with PBS for the injected solution (0.25 mg/ml in PBS plus 0.1% DMSO). The control group was treated with an equal amount of 0.1% DMSO in PBS. BrdU was injected similarly as described above. At P7, the mice were euthanized, and the eyes were enucleated. Mice were housed under specific-pathogen free conditions. SUPM2 or Karpas 299 cells (2 × 106) were injected subcutaneously in immunodeficient NSG mice or Rag2/Il2rg immunodeficient mice with transgenic expression of humanized CSF1 ligand(48). Upon injections, mice were fed with either nutritionally complete pexidartinib-containing (275mg PLX3397/kg) or control chow, provided under MTA by Plexxikon. Toxicity secondary to drug delivery was assessed by daily monitoring of clinical condition (appearance, activity and body condition). Tumors were measured and mice humanely euthanized approximately 14 days after pexidartinib- or control-treatment. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following administration of single doses in healthy subjects and multiple doses in patients, the mean Cmax was 8625 ng/mL and the mean AUC was 77465 ngxh/mL. The median Tmax was 2.5 hours and the time to reach the steady state was approximately 7 days. Administration of pexidartinib with a high fat meal resulted in an increased drug Cmax and AUC by 100%, with a delay in Tmax by 2.5 hours. Pexidartinib is predominantly excreted via feces, where fecal excretion accounts for 65% of total pexidartinib elimination. Via this route of elimination, about 44% of the compound found in feces is recovered as unchanged parent drug. The renal elimination accounts for 27% of pexidartinib elimination, where more than 10% of the compound is found as the N-glucuronide metabolite. The apparent volume of distribution of pexidartinib is about 187 L. In rats, pexidartinib was shown to penetrate into the central nervous system. The apparent clearance is about 5.1 L/h. Metabolism / Metabolites Pexidartinib primarily undergoes oxidation mediated by hepatic CYP3A4 and glucuronidation by UGT1A4. Following UGT1A4-mediated glucuronidation, a major inactive N-glucuronide metabolite is formed with approximately 10% higher exposure than the parent drug after a single dose administration of pexidartinib. Based on the findings of _in vitro_ studies, CYP1A2 and CYP2C9 may also play a minor role in drug metabolism. Biological Half-Life The elimination half-life is about 26.6 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Elevations in serum aminotransferase levels are common during pexidartinib therapy, occurring in 50% to 90% of patients and rising above 5 times the upper limit of the normal range in 12% to 20%. In addition, elevations in alkaline phosphatase levels occur in up to 20% of treated persons. In registration trials, clinically apparent liver injury with jaundice developed in 5% of patients. The time to onset of liver injury was typically between 2 and 6 weeks, and the pattern of liver enzyme elevations was mixed or cholestatic. Autoimmune and immune-allergic features were not prominent. Liver biopsy demonstrated bile duct injury and loss, and at least 3 patients in studies for conditions other than TGCT developed bile duct paucity and features of vanishing bile duct syndrome that ultimately led to liver transplantation in one subject. Pexidartinib has had limited clinical use and the frequency and spectrum of acute liver injury with its use is not yet well defined. Likelihood score: B (likely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of pexidartinib during breastfeeding. Because pexidartinib is over 99% bound to plasma proteins, the amount in milk is likely to be low. However, the manufacturer recommends that breastfeeding be discontinued during pexidartinib therapy and for 1 week after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Based on the findings of _in vitro_ plasma protein binding study, pexidartinib is about 99% bound to serum proteins, where it is extensively bound to human serum albumin by 99.9% and alpha-1-acid glycoprotein by 89.9%. LiverTox Summary Pexidartinib is an orally available small molecule multi-kinase inhibitor that is used as an antineoplastic agent in the treatment of tenosynovial giant cell tumors. Pexidartinib is associated with a high rates of serum aminotransferase and alkaline phosphatase elevations during therapy and has been implicated in several cases of clinically apparent liver injury marked by progressive intrahepatic bile duct injury, some of which resulted in liver transplantation or were fatal. |
| 参考文献 |
|
| 其他信息 |
Pharmacodynamics
Pexidartinib works by suppressing the growth of tenosynovial giant cell tumors. In clinical trials comprising of patients with symptomatic tenosynovial giant cell tumor, pexidartinib had a higher overall response rate, characterized by improved patient symptoms and functional outcomes, compared to placebo. Pexidartinib works by inhibiting the activation and signaling of tumor-permissive cytokines and receptor tyrosine kinases that play a central role in tumor cell proliferation and survival. Taking pexidartinib with a high-fat meal may increase the incidence and severity of adverse reactions, including hepatotoxicity. Pexidartinib is a pyrrolopyridine that is 5-chloro-1H-pyrrolo[2,3-b]pyridine which is substituted by a [6-({[6-(trifluoromethyl)pyridin-3-yl]methyl}amino)pyridin-3-yl]methyl group at position 3. It is a potent multi-targeted receptor tyrosine kinase inhibitor of CSF-1R, KIT, and FLT3 (IC50 of 20 nM, 10 nM and 160 nM, respectively). Approved by the FDA for the treatment of adult patients with symptomatic tenosynovial giant cell tumor (TGCT). It has a role as an EC 2.7.10.1 (receptor protein-tyrosine kinase) inhibitor and an antineoplastic agent. It is a pyrrolopyridine, an organochlorine compound, an aminopyridine, an organofluorine compound and a secondary amino compound. Pexidartinib is a selective tyrosine kinase inhibitor that works by inhibiting the colony-stimulating factor (CSF1)/CSF1 receptor pathway. Pexidartinib was originally developed by Daiichi Sankyo, Inc. and it was approved by the FDA in August 2019 as the first systemic therapy for adult patients with symptomatic tenosynovial giant cell tumor. Tenosynovial giant cell tumor is a rare form of non-malignant tumor that causes the synovium and tendon sheaths to thicken and overgrow, leading to damage in surrounding joint tissue. Debilitating symptoms often follow with tenosynovial giant cell tumors, along with a risk of significant functional limitations and a reduced quality of life in patients. While surgical resection is a current standard of care for tenosynovial giant cell tumor, there are tumor types where surgeries are deemed clinically ineffective with a high risk of lifetime recurrence. Pexidartinib works by blocking the immune responses that are activated in tenosynovial giant cell tumors. In clinical trials, pexidartinib was shown to promote improvements in patient symptoms and functional outcomes in TGCT. Pexidartinib is available in oral formulations and it is commonly marketed as Turalio. Pexidartinib is a Kinase Inhibitor. The mechanism of action of pexidartinib is as a Kinase Inhibitor, and Tyrosine Kinase Inhibitor, and Colony Stimulating Factor Receptor Type 1 (CSF-1R) Inhibitor, and Cytochrome P450 3A Inducer, and Cytochrome P450 2B6 Inhibitor, and UGT1A1 Inhibitor. Pexidartinib is an orally available small molecule multi-kinase inhibitor that is used as an antineoplastic agent in the treatment of tenosynovial giant cell tumors. Pexidartinib is associated with a high rates of serum aminotransferase and alkaline phosphatase elevations during therapy and has been implicated in several cases of clinically apparent liver injury marked by progressive intrahepatic bile duct injury, some of which resulted in liver transplantation or were fatal. Pexidartinib is a small-molecule receptor tyrosine kinase (RTK) inhibitor of proto-oncogene receptor tyrosine kinase (KIT), colony-stimulating factor-1 receptor (CSF1R) and FMS-like tyrosine kinase 3 (FLT3), with antineoplastic activity. Upon oral administration, pexidartinib targets, binds to and inhibits phosphorylation of KIT, CSF1R and FLT3 harboring an internal tandem duplication (ITD) mutation. This results in the inhibition of tumor cell proliferation. FLT3, CSF1R and FLT3 are overexpressed or mutated in many cancer cell types and play major roles in tumor cell proliferation and metastasis. PEXIDARTINIB is a small molecule drug with a maximum clinical trial phase of IV (across all indications) that was first approved in 2019 and is indicated for neoplasm and tenosynovial giant cell tumor and has 16 investigational indications. This drug has a black box warning from the FDA. Immune-regulated pathways influence multiple aspects of cancer development. In this article we demonstrate that both macrophage abundance and T-cell abundance in breast cancer represent prognostic indicators for recurrence-free and overall survival. We provide evidence that response to chemotherapy is in part regulated by these leukocytes; cytotoxic therapies induce mammary epithelial cells to produce monocyte/macrophage recruitment factors, including colony stimulating factor 1 (CSF1) and interleukin-34, which together enhance CSF1 receptor (CSF1R)-dependent macrophage infiltration. Blockade of macrophage recruitment with CSF1R-signaling antagonists, in combination with paclitaxel, improved survival of mammary tumor-bearing mice by slowing primary tumor development and reducing pulmonary metastasis. These improved aspects of mammary carcinogenesis were accompanied by decreased vessel density and appearance of antitumor immune programs fostering tumor suppression in a CD8+ T-cell-dependent manner. These data provide a rationale for targeting macrophage recruitment/response pathways, notably CSF1R, in combination with cytotoxic therapy, and identification of a breast cancer population likely to benefit from this novel therapeutic approach. Significance: These findings reveal that response to chemotherapy is in part regulated by the tumor immune microenvironment and that common cytotoxic drugs induce neoplastic cells to produce monocyte/macrophage recruitment factors, which in turn enhance macrophage infiltration into mammary adenocarcinomas. Blockade of pathways mediating macrophage recruitment, in combination with chemotherapy, significantly decreases primary tumor progression, reduces metastasis, and improves survival by CD8+ T-cell-dependent mechanisms, thus indicating that the immune microenvironment of tumors can be reprogrammed to instead foster antitumor immunity and improve response to cytotoxic therapy. [1] Purpose: In mice, retinal development continues throughout the postnatal stage accompanied by the proliferation of retinal precursor cells. Previous reports showed that during the postnatal stage microglia increase from postnatal day 0 (P0) to P7. However, how microglia are associated with retinal development remains unknown. Methods: The involvement of microglia in retinal development was investigated by two approaches, microglial activation and loss, using lipopolysaccharide (LPS) and PLX3397 (Pexidartinib), respectively. Results: LPS injection at 1 mg/kg, intraperitoneally (i.p.) in the neonatal mice increased the number of retinal microglia at P7. 5-Bromo-2´-deoxyuridine (BrdU)-positive proliferative cells were increased by LPS treatment compared to the control group. The proliferative cells were mainly colocalized with paired box 6 (Pax6), a marker of retinal precursor cells. However, the depletion of microglia by treatment with PLX3397 decreased the BrdU-positive proliferative cells. Moreover, progranulin deficiency decreased the number of microglia and retinal precursor cells. Conclusions: These findings indicated that microglia regulate the proliferation of immature retinal cells. [2] Background Pexidartinib, a novel, orally administered small-molecule tyrosine kinase inhibitor, has strong selectivity against colony-stimulating factor 1 receptor. This phase I, nonrandomized, open-label multiple-dose study evaluated pexidartinib safety and efficacy in Asian patients with symptomatic, advanced solid tumors. Materials and Methods Patients received pexidartinib: cohort 1, 600 mg/d; cohort 2, 1000 mg/d for 2 weeks, then 800 mg/d. Primary objectives assessed pexidartinib safety and tolerability, and determined the recommended phase 2 dose; secondary objectives evaluated efficacy and pharmacokinetic profile. Results All 11 patients (6 males, 5 females; median age 64, range 23-82; cohort 1 n = 3; cohort 2 n = 8) experienced at least one treatment-emergent adverse event; 5 experienced at least one grade ≥ 3 adverse event, most commonly (18%) for each of the following: increased aspartate aminotransferase, blood alkaline phosphatase, gamma-glutamyl transferase, and anemia. Recommended phase 2 dose was 1000 mg/d for 2 weeks and 800 mg/d thereafter. Pexidartinib exposure, area under the plasma concentration-time curve from zero to 8 h (AUC0-8h), and maximum observed plasma concentration (Cmax) increased on days 1 and 15 with increasing pexidartinib doses, and time at Cmax (Tmax) was consistent throughout all doses. Pexidartinib exposure and plasma levels of adiponectin and colony-stimulating factor 1 increased following multiple daily pexidartinib administrations. One patient (13%) with tenosynovial giant cell tumor showed objective tumor response. Conclusions This was the first study to evaluate pexidartinib in Asian patients with advanced solid tumors. Pexidartinib was safe and tolerable in this population at the recommended phase 2 dose previously determined for Western patients (funded by Daiichi Sankyo; clinicaltrials.gov number, NCT02734433).[3] Background and objectives: Excessive adipose tissue macrophage accumulation in obesity has been implicated in mediating inflammatory responses that impair glucose homeostasis and promote insulin resistance. Colony-stimulating factor 1 (CSF1) controls macrophage differentiation, and here we sought to determine the effect of a CSF1 receptor inhibitor, Pexidartinib/PLX3397, on adipose tissue macrophage levels and understand the impact on glucose homeostasis in mice. Methods: A Ten-week-old mice were fed a chow or high-fat diet for 10 weeks and then treated with PLX3397 via oral gavage (50 mg/kg) every second day for 3 weeks, with subsequent monitoring of glucose tolerance, insulin sensitivity and assessment of adipose tissue immune cells. Results: PLX3397 treatment substantially reduced macrophage numbers in adipose tissue of both chow and high-fat diet fed mice without affecting total myeloid cell levels. Despite this, PLX3397 did not greatly alter glucose homeostasis, did not affect high-fat diet-induced increases in visceral fat cytokine expression (Il-6 and Tnfa) and had limited effect on the phosphorylation of the stress kinases JNK and ERK and macrophage polarization. Conclusions: Our results indicate that macrophage infiltration of adipose tissue induced by a high-fat diet may not be the trigger for impairments in whole body glucose homeostasis, and that anti-CSF1 therapies are not likely to be useful as treatments for insulin resistance. [4] Purpose: Peripheral T-cell lymphomas are clinically aggressive and usually fatal, as few complete or durable remissions are achieved with currently available therapies. Recent evidence supports a critical role for lymphoma-associated macrophages during T-cell lymphoma progression, but the specific signals involved in the cross-talk between malignant T-cells and their microenvironment are poorly understood. Colony-stimulator factor 1 receptor (CSF1R, CD115) is required for the homeostatic survival of tissue-resident macrophages. Interestingly, it’s aberrant expression has been reported in a subset of tumors. In this manuscript we evaluated its expression and oncogenic role in T-cell lymphomas. Experimental Design: Loss-of-function studies, including pharmacologic inhibition with a clinically available tyrosine-kinase inhibitor, Pexidartinib, were performed in multiple in vitro and in vivo models. In addition, proteomic and genomic screenings were performed to discover signaling pathways that are activated downstream of CSF1R signaling. Results: We observed that CSF1R is aberrantly expressed in many T-cell lymphomas, including a significant number of peripheral and cutaneous T-cell lymphomas. Colony-stimulating factor 1 (CSF1), in an autocrine or paracrine-dependent manner, leads to CSF1R autophosphorylation and activation in malignant T-cells. Furthermore, CSF1R signaling was associated with significant changes in gene expression and in the phosphoproteome, implicating PI3K/AKT/mTOR in CSF1R-mediated T-cell lymphoma growth. We also demonstrated that inhibition of CSF1R in-vivo and in-vitro models is associated with decreased T-cell lymphoma growth. Conclusions: Collectively, these findings implicate CSF1R in T-cell lymphomagenesis and have significant therapeutic implications. [5] |
| 分子式 |
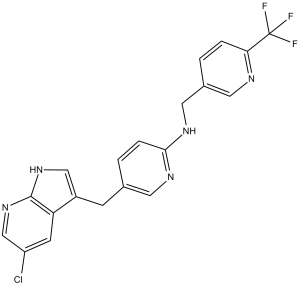
C20H15CLF3N5
|
|---|---|
| 分子量 |
417.81
|
| 精确质量 |
417.096
|
| 元素分析 |
C, 57.49; H, 3.62; Cl, 8.49; F, 13.64; N, 16.76
|
| CAS号 |
1029044-16-3
|
| 相关CAS号 |
Pexidartinib hydrochloride;2040295-03-0
|
| PubChem CID |
25151352
|
| 外观&性状 |
Yellow solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
580.0±50.0 °C at 760 mmHg
|
| 闪点 |
304.6±30.1 °C
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
| 折射率 |
1.662
|
| LogP |
4.77
|
| tPSA |
66.49
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
29
|
| 分子复杂度/Complexity |
537
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC1C([H])=NC2=C(C=1[H])C(=C([H])N2[H])C([H])([H])C1=C([H])N=C(C([H])=C1[H])N([H])C([H])([H])C1=C([H])N=C(C(F)(F)F)C([H])=C1[H]
|
| InChi Key |
JGWRKYUXBBNENE-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C20H15ClF3N5/c21-15-6-16-14(10-28-19(16)29-11-15)5-12-2-4-18(26-7-12)27-9-13-1-3-17(25-8-13)20(22,23)24/h1-4,6-8,10-11H,5,9H2,(H,26,27)(H,28,29)
|
| 化学名 |
5-[(5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)methyl]-N-[[6-(trifluoromethyl)pyridin-3-yl]methyl]pyridin-2-amine
|
| 别名 |
Pexidartinib; CML-261; FP-113; PLX3397; PLX 3397; CML 261; CML261; PLX-3397;FP 113; FP113; Pexidartinib (PLX3397); CML-261; Pexidartinib [INN]; trade name: Turalio
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 5 mg/mL (11.97 mM) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.98 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.98 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.08 mg/mL (4.98 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,您可以将 100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 配方 5 中的溶解度: 10% DMSO+40% PEG 300+ddH2O:15 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3934 mL | 11.9672 mL | 23.9343 mL | |
| 5 mM | 0.4787 mL | 2.3934 mL | 4.7869 mL | |
| 10 mM | 0.2393 mL | 1.1967 mL | 2.3934 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02975700 | Active Recruiting |
Drug: PLX3397 | Melanoma | Daiichi Sankyo Co., Ltd. | January 2017 | Not Applicable |
| NCT04488822 | Active Recruiting |
Drug: Pexidartinib | Tenosynovial Giant Cell Tumor | Daiichi Sankyo Co., Ltd. | September 25, 2020 | Phase 3 |
| NCT04703322 | Recruiting | Drug: Pexidartinib | Tenosynovial Giant Cell Tumor | Daiichi Sankyo Co., Ltd. | March 15, 2021 | Phase 2 |
| NCT04635111 | Recruiting | Drug: TURALIO™ | Hepatotoxicity Tenosynovial Giant Cell Tumor |
Daiichi Sankyo, Inc. | January 7, 2021 | |
| NCT02390752 | Recruiting | Drug: Turalio | Sarcoma Neurofibroma, Plexiform |
National Cancer Institute (NCI) |
April 29, 2015 | Phase 1 |
Combined PLX3397 and PTX treatment inhibits metastasis in a CD8-dependent manner. Cancer Discov. 2011 Jun 1; 1: 54–67. |
PTX in combination with PLX3397 induces antitumor T-cell response. Cancer Discov. 2011 Jun 1; 1: 54–67. |
CD68/CD4/CD8 immune signature is an independent prognostic indicator of breast cancer survival. Cancer Discov. 2011 Jun;1(1):54-67. |