| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
Glucagon receptor (GCGR)
|
|---|---|
| 体外研究 (In Vitro) |
PF-06291874是一种有效的选择性竞争性人GCGR拮抗剂[平衡解离常数,140 nM (70.5 ng/ml)]。动力学分析表明,PF-06291874具有快速的开关速率。PF-06291874与人血浆蛋白高度结合,平均游离分数为0.55%。[3]
PF-06291874 暴露的半衰期约为 19.7-22.7 小时,并且大致与剂量成比例。 PF-06291874的速率开关速度快。 PF-06291874 的平均游离分数约为 0.55%,与人血浆蛋白强烈结合[3]。 |
| 体内研究 (In Vivo) |
PF-06291874 的暴露量大约与剂量成比例,半衰期为 ∼19.7-22.7 小时。 PF-06291874 具有快速的开启和关闭速率。 PF-06291874 与人血浆蛋白高度结合,平均游离分数为 ∼0.55%[3]。
PF-06291874暴露与剂量成正比,半衰期约为19.7-22.7小时。第14天,空腹血糖和平均每日血糖值以剂量依赖的方式从基线降低,在150mg剂量下,安慰剂校正后分别降低34.3和42.4 mg/dl。MMTT后,观察到胰高血糖素和总胰高血糖素样肽-1 (GLP-1)的剂量依赖性增加,尽管胰岛素、c肽或活性GLP-1水平没有明显变化。观察到低密度脂蛋白胆固醇的小剂量依赖性增加,以及血清转氨酶的可逆性增加,这些增加基本上在实验室参考范围内。在第2天和第14天也观察到循环糖异生氨基酸的增加。所有剂量水平的PF-06291874均具有良好的耐受性。 结论:PF-06291874具有良好的耐受性,具有适合每日一次给药的药代动力学特征,并且在低血糖风险最小的情况下降低血糖。 |
| 酶活实验 |
MDpocket检测MD轨迹中的口袋[1]
MDpocket是一个开源工具,用于检测MD模拟轨迹中的潜在结合口袋。在使用MDpocket之前,有必要使用GROMACS 2020从处理过的MD轨迹中每100 ps提取一个PDB文件。然后通过MDpocket检测PDB结构集合,以输出整个轨迹中存在的凹坑,这些凹坑能够通过可视化软件PyMOL进行观察(http://www.pymol.org). 该分析沿着GCGR/胰高血糖素系统的五条轨迹进行。MDpocket还允许我们计算所选口袋的体积。 分子对接[1] 为了研究已知活性分子与GCGR之间的相互作用模式,使用AutoDock Tools软件包进行了柔性对接。优化受体和配体以分别产生相应的低能3D构象和相应的电离态(pH 7.0)。从动态构象和晶体结构中发现的结合位点被用作对接网格中心,口袋周围的残基被设置为翻转。然后将制备的化合物对接到GCGR袋中,并输出每个配体的前20种构象。根据相互作用能和目视检查选择最合适的结合构象。所有结果均使用PyMOL进行分析和可视化(http://www.pymol.org). 为了模拟更真实的生理环境,在口袋2、口袋4和口袋5周围添加了POPC磷脂分子。 |
| 动物实验 |
This randomized, double-blind, placebo-controlled, multiple dose-escalating study of oral PF-06291874 (ClinicalTrials.gov: NCT01856595) was conducted at three clinical research centres in the USA.
Part A was conducted in cohorts of patients receiving either 5, 15, 50, 100 or 150 mg of PF-06291874 or placebo once-daily; and Part B, in cohorts of patients receiving either 15 or 30 mg of PF-06291874 or placebo once-daily. Patients in the 5-, 15-, 50- and 150-mg cohorts in Part A, and the 15-mg cohort in Part B received PF-06291874 for 14 days in an inpatient setting. Patients in the 100-mg cohort (Part A) and the 30-mg cohort (Part B) received PF-06291874 for 28 days (14 days inpatient; 14 days outpatient). The daily dose of 150 mg in Part A of the study was expected to achieve plasma concentrations resulting in mean receptor antagonism of 90% over the course of the day, based on a functional in vitro binding constant in cells expressing the human glucagon receptor. The 30-mg dose was selected for Part B after the assessment of safety and glucose-lowering data from the 15-mg cohort in Part B. PF-06291874 was administered daily before breakfast. Standardized meals were provided while in the clinical research unit. A mixed-meal tolerance test (MMTT; 700-kcal Ensure Plus®) was given in place of breakfast on days −1, 14 and 28 (in applicable cohorts) to assess attenuation by PF-06291874 of glucose excursion, and to measure other biomarkers after controlled intake of nutrients and energy. Doses of PF-06291874 or placebo were administered on days 14 and 28 immediately before the MMTT. Escalation to the next dose level in both parts occurred in the absence of dose-limiting toxicity or other significant safety findings.[3]
|
| 药代性质 (ADME/PK) |
Pharmacokinetic Results [3]
All patients who received PF-06291874, except two (one with insufficient samples collected owing to study discontinuation on day 14, and one dosing error in which the patient received PF-06291874 instead of placebo for ∼5 days), were included in the pharmacokinetic analysis. PF-06291874 plasma exposure (Cmax and AUCτ (area under the concentration-time profile from time zero to time tau (τ)) increased proportionally throughout the dose range studied (Table S1). Dose-normalized pharmacokinetic variables were similar between patients receiving PF-06291874 on a background of metformin, or metformin and sulphonylurea. Accumulation ratios of plasma exposure after 14 days of dosing ranged from 1.5- to 2-fold relative to day 1; and steady-state concentrations were reached within 7 days of first dose. The median time to maximum drug concentration ranged from 4 to 6 h; and the mean apparent terminal elimination t½ on day 14 ranged from 19.7 to 22.7 h and was independent of dose. Urinary recovery of unchanged PF-06291874 was minimal (<0.3% of the administered dose when quantifiable). |
| 毒性/毒理 (Toxicokinetics/TK) |
Safety and Tolerability [3]
PF-06291874 was generally well tolerated; there were no deaths, severe AEs, permanent discontinuations, dose reductions because of treatment-emergent AEs, or clinically significant variations in vital signs (Table S5) or ECG variables. There was one temporary discontinuation as a result of chromatopsia (the patient discontinued on day 8 and resumed PF-06291874, 30 mg, on day 11), which was considered mild in severity by the investigator. In patients on background metformin, the incidence of hypoglycaemia (fasting blood glucose ≤70 mg/dl) was approximately the same across all doses of PF-06291874 and similar to that in the placebo group. In patients on background metformin and sulphonylurea, the incidence was similar in subjects receiving placebo and 15 mg PF-06291874, but higher in patients receiving 30 mg PF-06291874. None of the hypoglycaemia AEs were considered severe and recovery was rapid. With the exception of hypoglycaemia AEs, there was no dose-related increase in AE frequency. The most commonly reported AEs were diarrhoea, nausea, upper respiratory infection and headache. All were judged to be mild by the investigator. After 14 days of dosing, there appeared to be a dose-dependent increase in mean fasting LDL cholesterol values. Patients who received the 150-mg dose had a mean increase from baseline of ∼10% (∼20% compared with placebo-treated subjects; Figure 2). An increase of similar magnitude in total cholesterol was observed; however, changes in HDL cholesterol, triglycerides and apolipoprotein B100 were more variable (Table S6). Small, dose-related, reversible increases in mean alanine aminotransferase (ALT) and aspartate aminotransferase (AST) values were observed (Figure 3) within the first few days of dosing, remaining constant or decreasing slightly over 14/28 days of dosing. The majority of observed aminotransferase increases remained within the laboratory reference range and returned to baseline values after discontinuation of dosing. None of these changes was associated with an increase in bilirubin. Three of the 86 patients who received PF-06291874 had ALT or AST elevations >3× ULN. One patient who received 100 mg on background metformin had three consecutive ALT values of >3× ULN (peak value 105 IU/l) beginning 7 days after the start of dosing. The ALT values returned to approximate baseline values by day 21, while the subject continued to receive PF-06291874. A second subject with an ALT value of >3× ULN had received 30 mg in combination with metformin/sulphonylurea. After 28 days of dosing, the subject's ALT value was 81 IU/l (baseline 49 IU/l). At the follow-up visit, 8 days after the last dose, the subject's ALT value remained elevated at 87 IU/l. A third subject who received 15 mg in combination with metformin/sulphonylurea had a single AST value >3× ULN (105 IU/l) on day 14. This value represented a slight increase from the baseline value of 87 IU/l. A repeat assessment was performed on day 17 (3 days after the final dose), at which time the AST value was 52 IU/l. |
| 参考文献 |
|
| 其他信息 |
PF-06291874 is under investigation in clinical trial NCT02175121 (Safety, Tolerability, Pharmacokinetics And Pharmacodynamics Study of PF-06291874 as Oral Monotherapy To Treat Adults With Type 2 Diabetes Mellitus).
Consistent with studies in healthy subjects, plasma concentrations of PF-06291874 increased in a dose-proportional manner across the studied range and the pharmacokinetic profile supports once-daily dosing. PF-06291874 was well tolerated; the maximum tolerated dose was not identified. Although this study was not optimally designed to establish the effects of PF-06291874 on plasma lipids, there was an ∼20% (placebo-corrected) increase in plasma LDL cholesterol relative to baseline at the highest dose tested. A trend for increased plasma LDL cholesterol was also identified in the MK-0893 12-week study and in the LY2409021 28-day study, indicating that this could be a mechanism-related effect. Indeed, results from investigation of MK-0893 in a preclinical mouse model, coupled with archived clinical samples from the 12-week study of MK-0893, suggest that glucagon receptor antagonists may increase LDL cholesterol by increasing cholesterol absorption from the gut. Data from the present study, not included in this manuscript, showed that there was no change in mevalonic acid concentrations, suggesting glucagon receptor antagonism has no effect on cholesterol biosynthesis. Similar to other glucagon receptor antagonists, small dose-related increases in ALT and AST were observed in patients treated with PF-06291874. These changes were not associated with any changes in bilirubin or alkaline phosphatase. The mechanism underlying these increases is not understood, although it did not appear to be associated with elevated plasma alanine concentrations; there were no obvious other reasons (alcohol use, concomitant medications or infections) for these observed increases. Further work will be necessary to determine whether increases in AST and ALT are a physiological adaptation to blocking glucagon signalling. In summary, once-daily dosing with the glucagon receptor antagonist, PF-06291874, elicited reductions in fasting and postprandial glucose with a low risk of hypoglycaemia in patients on background therapy of either metformin alone, or in combination with a sulphonylurea. Mild, reversible increases in serum aminotransferase levels were observed, along with mild increases in LDL cholesterol. Administration of PF-06241874 for up to 4 weeks was generally well tolerated, supporting its suitability for continued clinical development in longer-term studies.[3] |
| 分子式 |
C26H28F3N3O4
|
|---|---|
| 分子量 |
503.5134
|
| 精确质量 |
503.203
|
| 元素分析 |
C, 62.02; H, 5.61; F, 11.32; N, 8.35; O, 12.71
|
| CAS号 |
1393124-08-7
|
| PubChem CID |
60151939
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
675.0±55.0 °C at 760 mmHg
|
| 闪点 |
362.0±31.5 °C
|
| 蒸汽压 |
0.0±2.2 mmHg at 25°C
|
| 折射率 |
1.563
|
| LogP |
5.44
|
| tPSA |
93.4
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
36
|
| 分子复杂度/Complexity |
718
|
| 定义原子立体中心数目 |
1
|
| SMILES |
FC(C1C([H])=NN(C=1[H])C1C(C([H])([H])[H])=C([H])C(=C([H])C=1C([H])([H])[H])O[C@]([H])(C1C([H])=C([H])C(C(N([H])C([H])([H])C([H])([H])C(=O)O[H])=O)=C([H])C=1[H])C([H])([H])C([H])([H])C([H])([H])[H])(F)F
|
| InChi Key |
IBDYYOQKQCCSDP-QFIPXVFZSA-N
|
| InChi Code |
InChI=1S/C26H28F3N3O4/c1-4-5-22(18-6-8-19(9-7-18)25(35)30-11-10-23(33)34)36-21-12-16(2)24(17(3)13-21)32-15-20(14-31-32)26(27,28)29/h6-9,12-15,22H,4-5,10-11H2,1-3H3,(H,30,35)(H,33,34)/t22-/m0/s1
|
| 化学名 |
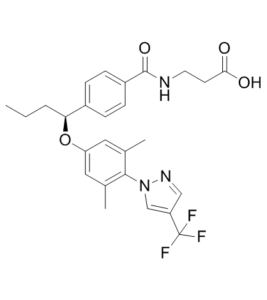
3-[[4-[(1S)-1-[3,5-dimethyl-4-[4-(trifluoromethyl)pyrazol-1-yl]phenoxy]butyl]benzoyl]amino]propanoic acid
|
| 别名 |
PF6291874; PF-06291874; 1393124-08-7; glucagon receptor antagonists-4; PF-06291874; CGY4I8F278; (S)-3-(4-(1-(3,5-dimethyl-4-(4-(trifluoromethyl)-1H-pyrazol-1-yl)phenoxy)butyl)benzamido)propanoic acid; UNII-CGY4I8F278; CHEMBL2381848; .BETA.-ALANINE, N-(4-((1S)-1-(3,5-DIMETHYL-4-(4-(TRIFLUOROMETHYL)-1H-PYRAZOL-1-YL)PHENOXY)BUTYL)BENZOYL)-; PF-6291874; PF 6291874; PF 06291874; PF06291874
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~100 mg/mL (~198.6 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.13 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.13 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.13 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9861 mL | 9.9303 mL | 19.8606 mL | |
| 5 mM | 0.3972 mL | 1.9861 mL | 3.9721 mL | |
| 10 mM | 0.1986 mL | 0.9930 mL | 1.9861 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01794364 | Completed | Drug: PF-06291874 Other: placebo |
Healthy | Pfizer | January 2013 | Phase 1 |
| NCT01856595 | Completed | Drug: PF-06291874 Drug: Placebo |
Diabetes Mellitus, Type 2 | Pfizer | May 13, 2013 | Phase 1 |
| NCT02554877 | Completed | Drug: PF-06291874 Drug: Placebo |
Type 2 Diabetes Mellitus | Pfizer | October 2015 | Phase 2 |
| NCT02175121 | Completed | Drug: PF-06291874 Drug: Placebo |
Diabetes Mellitus, Type II | Pfizer | August 2014 | Phase 2 |
| NCT01499017 | Terminated | Drug: PF-06291874 or placebo Drug: PF-06291874 or Placebo |
Healthy | Pfizer | November 2011 | Phase 1 |