| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
p110α (IC50 = 2 nM); p110β (IC50 = 3 nM); p110δ (IC50 = 3 nM); p110γ (IC50 = 15 nM); mTORC1 (IC50 = 20 nM); mTORC2 (IC50 = 83 nM); DNA-PK (IC50 = 23 nM)
|
|---|---|
| 体外研究 (In Vitro) |
PI-103 有效抑制蛋白激酶 mTOR 的雷帕霉素敏感 (mTORC1) 和雷帕霉素不敏感 (mTORC2) 复合物。[1] PI-103 阻断 PI3K/Akt 激活,这种激活既是内在发生的,也是由生长因子诱导的。 [2] 在母细胞中,PI-103 可防止白血病增殖,防止白血病祖细胞克隆,并引发线粒体凋亡,特别是在白血病干细胞所在的室中。 PI-103 抑制 p110α 的效果比 p110 的抑制效果高 200 倍以上。此外,PI-103 还可有效抑制肌管和脂肪细胞中 PIP3 和 PI(3,4)P2 的合成。 [2] PI-103 抑制 Akt 磷酸化,其 IC95 比 LY294002 低 100 倍。令人惊讶的是,PI-103 完全保护动物免受胰岛素引起的血糖下降。在母细胞和未成熟白血病细胞中,PI-103 具有与依托泊苷相加的促凋亡作用。 [2]
|
| 体内研究 (In Vivo) |
当肿瘤达到 50-100 mm3 时,将动物随机分组并用载体或 PI-103 进行治疗。 18 天后,PI-103 显示出显着的活性,肿瘤大小平均缩小 4 倍。 [2] 在病前(根据体重、食物和水的消耗量、活动水平和一般检查)或尸检时,用 PI-103 治疗的小鼠没有表现出明显的毒性作用。正如 p110α 和 mTOR 阻断所预期的那样,治疗后的肿瘤显示出较低水平的磷酸化 Akt 和 S6。 PI-103 治疗对神经胶质瘤异种移植物具有细胞抑制作用。 [2]
|
| 酶活实验 |
使用脂质激酶活性的标准薄层色谱 (TLC) 测定或高通量膜捕获测定来测量 IC50 值。通过制备含有激酶、抑制剂(2% DMSO 最终浓度)、缓冲液(25 mM HEPES,pH 7.4,10 mM MgCl2)和新鲜超声处理的磷脂酰肌醇(100 μg/mL)的反应混合物来进行激酶反应。通过添加含有 10 μCi γ-32P-ATP 的 ATP 至终浓度 10 或 100 μM 来引发反应,并在室温下进行 20 分钟。对于 TLC 分析,通过添加 105 μL 1N HCl,然后添加 160 μL CHCl3:MeOH (1:1) 来终止反应。将双相混合物涡旋,短暂离心,并使用预涂有 CHCl3 的凝胶加样移液器吸头将有机相转移至新管中。将该提取物点样在 TLC 板上,并在 65:35 正丙醇:1M 乙酸溶液中显色 3-4 小时。然后将 TLC 板干燥,暴露于磷光成像屏幕并定量。对于每种化合物,激酶活性通常在 10-12 个抑制剂浓度下测量,代表从测试的最高浓度 (100 μM) 稀释两倍。对于表现出显着活性的化合物,IC50 测定重复两到四次,报告值是这些独立测量值的平均值。
在 1 μmol/L ATP 存在下,使用闪烁邻近测定法测定磷脂酰肌醇 3-激酶抑制活性。 Invitrogen 基于 TR-FRET 的 LanthaScreen 技术用于确定 mTOR 蛋白激酶是否受到抑制。使用 GraphPad Prism 软件,在 1 mol/L ATP 存在下,计算最大浓度为 10 mol/L 的每种化合物的 IC50 值。[1] 蛋白激酶测定[1] 在含有25 mM HEPES、pH 7.4、10 mM MgCl2、200μM ATP(2.5μCi的γ-32P-ATP)和0.5 mg/mL BSA的测定中,对重组全长Abl或Abl(T315I)(Upstate)的Abl、Abl(T305I)抑制剂(终浓度:10μM)进行三次测定。优化的Abl肽底物EAIYAPFAKK用作磷酸受体(200μM)。通过在磷酸纤维素片上点样终止反应,用0.5%磷酸洗涤(约6次,每次5-10分钟)。将纸张干燥,并通过磷化法对转移的放射性进行定量。[1] Akt1、Akt1(ΔPH)、Akt2、Akt2(ΔPH)[1] 在含有25 mM HEPES、PH 7.4、10 mM MgCl2、200μM ATP(2.5μCi的γ-32P-ATP)和0.5 mg/mL BSA的试验中,对重组全长Akt1、Akt2、Akt3、Akt1(ΔPH)或Akt2(ΔPH。通过在硝化纤维上点样终止反应,用1M NaCl/1%磷酸洗涤硝化纤维(约6次,每次5-10分钟)。将纸张干燥,并通过磷化法对转移的放射性进行定量。[1] 有关激酶活性测试的更多信息,请参阅本文的补充材料/SI (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2946820/). |
| 细胞实验 |
使用基于 Invitrogen TR-FRET 的 LanthaScreen 技术来评估 mTOR 蛋白激酶的抑制作用。为了计算 IC50 值,在 1 μmol/L ATP 存在下以最大浓度 10 mol/L 测试化合物。
将 PI-103 应用于 U87MG 细胞 24 小时。使用细胞毒性检测试剂盒和 LDH 活性的比色测定来测量细胞死亡。计算细胞死亡百分比(每个实验点三个12孔板的平均值)的计算方法为[(实验值-低对照)/(高对照-低对照)×100],其中低对照用DMSO处理,使用 Triton 进行高度控制(1% Triton X-100,30 分钟,37°)。
人肿瘤细胞系U87MG、PC3、SKOV-3、IGROV-1、Detroit 562、HCT116、SNUC2B和LoVo购自美国典型培养物保藏中心。所有癌症细胞系均在含有2 mmol/L谷氨酰胺、100 U/mL青霉素和100μg/mL链霉素的DMEM中生长,并在37°C的空气中添加10%胎牛血清和5%CO2。人脐静脉内皮细胞(混合)及其适当的生长培养基和补充剂购自TCS CellWorks。根据供应商的说明培养细胞,并在第3至8代使用。通过台盼蓝排斥法判断,细胞存活率通常>90%。所有细胞系经PCR检测均为支原体阴性。使用Alamar Blue或磺基罗丹明B测定法测定导致肿瘤细胞增殖抑制50%的GI50值(浓度),对于人脐血管内皮细胞,在连续暴露于化合物96小时后,通过碱性磷酸酶测定法测定GI50值。在该试验之前,抗生素已被移除。[3] PTEN状态在大多数细胞系中得到验证,包括U87MG和IGROV-1,通过内部蛋白质印迹法进行蛋白质表达,此外,PIK3CA状态通过测序进行实验确定或验证。在结肠癌癌症细胞系的情况下,PTEN状态再次通过Western印迹在实验室内部得到证实。此外,使用单核苷酸多态性(SNP)分析来确认基因型与癌症基因组计划宇宙数据库3中提供的基因型相匹配,随后从该数据库中获得了KRAS和PIK3CA突变状态的数据。[3] 细胞系的易位、免疫印迹和磷酸蛋白免疫测定[3] 叉头易位试验如前所述进行。免疫印迹按如下方式进行。将细胞以3至5×105个细胞/孔的速度接种在2 mL培养基中的6孔板中,使其附着过夜,并用磷脂酰肌醇3-激酶抑制剂处理指定时间。孵育期后,小心地从孔中取出培养基,并向每个孔中加入约150μL补充有蛋白酶抑制剂和磷酸酶抑制剂的冰冷细胞提取缓冲液。在4°C下以14000×g离心10分钟后收集细胞上清液,并使用BCA蛋白检测试剂盒定量其蛋白质浓度。 对于蛋白质印迹,通过SDS-PAGE分离30μg每种裂解物,电转移到硝化纤维膜上,并用特异性一抗和辣根过氧化物酶偶联的二抗进行检测。用增强化学发光试剂检测信号。甘油醛-3-磷酸脱氢酶用作负载对照。 |
| 动物实验 |
Mice: Males aged five to six months are subcutaneously injected with one million cells in PBS, either from the FVB/N strain or the nude BALB/c strain. Mice are given daily injections of 50 mg/kg sorafenib and/or 10 mg/kg or 70 mg/kg of PI-103 when the tumor has grown to between 50 and 100 mm3. The same amount of DMSO is administered to control mice. Every two days, mice weight and tumor size are measured. Tumors are removed from mice after they have died and processed.
Xenografts [2] U87MG:ΔEGFR cells (106) were injected subcutaneously just caudal to the left forelimb in 6- to 12-week-old Balbc nu/nu mice (Harlan Sprague-Dawley). Mice with established tumors (50–100 mm3) were randomly allocated to IP treatment with 5 mg/kg PI-103 in 50% DMSO, or 50% DMSO alone (control). Tumor diameters were measured with calipers at 4 day intervals, and volumes were calculated from five mice per data point (mm3 = width2 × length/2). At necropsy, gross and microscopic analyses of all organs were performed to assess toxicity. Xenograft Tumor Efficacy and Pharmacodynamic Studies [3] Two million U87MG human glioblastoma cells were injected s.c., bilaterally, into female 6- to 8-wk-old CrTac: Ncr-Fox1(nu) [Ncr] athymic mice bred in-house. PI-540 was prepared in sterile saline, PI-620 in sterile water, and GDC-0941 in 10% DMSO, 5% Tween 20, and 85% sterile saline. Compounds were dosed in 0.1 mL/10 g body weight of vehicle once or twice daily (PI-540 and PI-620 i.p.; GDC-0941 p.o.). Control animals received an equivalent volume of appropriate vehicle. Dosing for therapy studies commenced when solid tumors were well established (~5 mm mean diameter) and continued according to the schedule indicated in the figure legends. Tumors were measured across two perpendicular diameters, and volumes were calculated according to the formula: V = 4/3π [(d1 + d2) / 4]3 (29). Animals were weighed regularly and observed for adverse effects. When the experiment was terminated, mice were bled, plasma samples prepared, and tumors excised and weighed. Values of the percentage treated/control (T/C) were calculated from the treated versus control final tumor weights. Tumor samples were snap frozen for pharmacokinetic and/or pharmacodynamic analysis at intervals after the last dose. For dedicated pharmacodynamic studies, animals were dosed for 4 d and samples obtained as before. Plasma and tumor samples were analyzed for compound concentrations and tumor samples assessed for evidence of biomarker modulation by Meso Scale Discovery electrochemiluminescence immunoassay and/or immunoblot, as previously described (29, 34). In some experiments, IGROV-1 human ovarian cancer xenografts were studied using similar methods to those for U87MG. |
| 药代性质 (ADME/PK) |
Pharmacokinetics of GDC-0941 [3]
The fast plasma and tissue clearance of PI-103 was the result of rapid glucuronidation of the phenol group. Despite decreases in mouse and human microsomal metabolism of PI-540 and PI-620 when compared with PI-103, significant in vivo glucuronidation was still observed. This accounts for the rapid clearance described in the previous section. To remove this metabolic liability, various phenol isosteres were synthesized and tested. The indazole derivative GDC-0941, which also contained the solubilizing sulfonyl piperazine, showed limited microsomal metabolism, resulting in 78% oral bioavailability, in addition to its potent inhibitory activity on the phosphatidylinositide 3-kinase pathway (Fig. 1B and E). Figure 6A shows the pharmacokinetics of GDC-0941 administered p.o. at 75 mg/kg to athymic mice bearing U87MG glioblastoma xenografts. GDC-0941 was very rapidly absorbed with Cmax achieved 30 minutes postadministration. Tumor distribution was equally rapid with Cmax reached at the same time. Although the tumor to plasma ratio was around 0.8, these properties resulted in tumor concentrations of compound well above the GI50 at 6 hours postadministration. |
| 参考文献 | |
| 其他信息 |
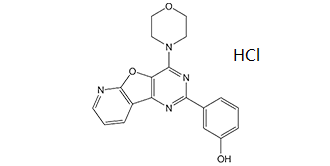
PI-103 is an organic heterotricyclic compound that is pyrido[3',2':4,5]furo[3,2-d]pyrimidine substituted at positions 2 and 4 by 3-hydroxyphenyl and morpholin-4-yl groups respectively. A dual-kinase inhibitor with anti-cancer properties. It has a role as an EC 2.7.1.137 (phosphatidylinositol 3-kinase) inhibitor, a mTOR inhibitor and an antineoplastic agent. It is a member of morpholines, a member of phenols, an organic heterotricyclic compound, a tertiary amino compound and an aromatic amine.
PI-103 is an inhibitor of p110α of class I PI3K.
Phosphoinositide 3-kinases (PI3-Ks) are an important emerging class of drug targets, but the unique roles of PI3-K isoforms remain poorly defined. We describe here an approach to pharmacologically interrogate the PI3-K family. A chemically diverse panel of PI3-K inhibitors was synthesized, and their target selectivity was biochemically enumerated, revealing cryptic homologies across targets and chemotypes. Crystal structures of three inhibitors bound to p110gamma identify a conformationally mobile region that is uniquely exploited by selective compounds. This chemical array was then used to define the PI3-K isoforms required for insulin signaling. We find that p110alpha is the primary insulin-responsive PI3-K in cultured cells, whereas p110beta is dispensable but sets a phenotypic threshold for p110alpha activity. Compounds targeting p110alpha block the acute effects of insulin treatment in vivo, whereas a p110beta inhibitor has no effect. These results illustrate systematic target validation using a matrix of inhibitors that span a protein family.[1] The PI3 kinase family of lipid kinases promotes cell growth and survival by generating the second messenger phosphatidylinositol-3,4,5-trisphosphate. To define targets critical for cancers driven by activation of PI3 kinase, we screened a panel of potent and structurally diverse drug-like molecules that target this enzyme family. Surprisingly, a single agent (PI-103) effected proliferative arrest in glioma cells, despite the ability of many compounds to block PI3 kinase signaling through its downstream effector, Akt. The unique cellular activity of PI-103 was traced directly to its ability to inhibit both PI3 kinase alpha and mTOR. PI-103 showed significant activity in xenografted tumors with no observable toxicity. These data demonstrate an emergent efficacy due to combinatorial inhibition of mTOR and PI3 kinase alpha in malignant glioma.[2] The phosphatidylinositide 3-kinase pathway is frequently deregulated in human cancers and inhibitors offer considerable therapeutic potential. We previously described the promising tricyclic pyridofuropyrimidine lead and chemical tool compound PI-103. We now report the properties of the pharmaceutically optimized bicyclic thienopyrimidine derivatives PI-540 and PI-620 and the resulting clinical development candidate GDC-0941. All four compounds inhibited phosphatidylinositide 3-kinase p110alpha with IC(50) < or = 10 nmol/L. Despite some differences in isoform selectivity, these agents exhibited similar in vitro antiproliferative properties to PI-103 in a panel of human cancer cell lines, with submicromolar potency in PTEN-negative U87MG human glioblastoma cells and comparable phosphatidylinositide 3-kinase pathway modulation. PI-540 and PI-620 exhibited improvements in solubility and metabolism with high tissue distribution in mice. Both compounds gave improved antitumor efficacy over PI-103, following i.p. dosing in U87MG glioblastoma tumor xenografts in athymic mice, with treated/control values of 34% (66% inhibition) and 27% (73% inhibition) for PI-540 (50 mg/kg b.i.d.) and PI-620 (25 mg/kg b.i.d.), respectively. GDC-0941 showed comparable in vitro antitumor activity to PI-103, PI-540, and PI-620 and exhibited 78% oral bioavailability in mice, with tumor exposure above 50% antiproliferative concentrations for >8 hours following 150 mg/kg p.o. and sustained phosphatidylinositide 3-kinase pathway inhibition. These properties led to excellent dose-dependent oral antitumor activity, with daily p.o. dosing at 150 mg/kg achieving 98% and 80% growth inhibition of U87MG glioblastoma and IGROV-1 ovarian cancer xenografts, respectively. Together, these data support the development of GDC-0941 as a potent, orally bioavailable inhibitor of phosphatidylinositide 3-kinase. GDC-0941 has recently entered phase I clinical trials.[3] |
| 分子式 |
C₁₉H₁₇CLN₄O₃
|
|---|---|
| 分子量 |
384.82
|
| 精确质量 |
384.099
|
| 元素分析 |
C, 59.30; H, 4.45; Cl, 9.21; N, 14.56; O, 12.47
|
| CAS号 |
371935-79-4
|
| 相关CAS号 |
PI-103;371935-74-9; 371935-79-4 (HCl)
|
| PubChem CID |
16739368
|
| 外观&性状 |
Light yellow to yellow solid
|
| LogP |
3.847
|
| tPSA |
84.51
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
489
|
| 定义原子立体中心数目 |
0
|
| SMILES |
Cl.O1CCN(C2=C3C(C4=CC=CN=C4O3)=NC(C3C=CC=C(C=3)O)=N2)CC1
|
| InChi Key |
XSQMYBFFYPTMFE-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C19H16N4O3.ClH/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23;/h1-6,11,24H,7-10H2;1H
|
| 化学名 |
3-(6-morpholin-4-yl-8-oxa-3,5,10-triazatricyclo[7.4.0.02,7]trideca-1(9),2(7),3,5,10,12-hexaen-4-yl)phenol;hydrochloride
|
| 别名 |
PI-103 hydrochloride; PI-103 HCl; PI 103 HYDROCHLORIDE; PI-103 Hydrochloride; 3-(6-morpholin-4-yl-8-oxa-3,5,10-triazatricyclo[7.4.0.02,7]trideca-1(9),2(7),3,5,10,12-hexaen-4-yl)phenol;hydrochloride; CHEMBL538346; PI 103 HCl; PI103 HCl
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 4.1~24 mg/mL (10.7~68.9 mM)
Ethanol: ~19.7 mg/mL (~60.2 mM) |
|---|
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5986 mL | 12.9931 mL | 25.9862 mL | |
| 5 mM | 0.5197 mL | 2.5986 mL | 5.1972 mL | |
| 10 mM | 0.2599 mL | 1.2993 mL | 2.5986 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。