| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
p110α (IC50 = 3 nM); p110β (IC50 = 33 nM); p110δ (IC50 = 3 nM); p110γ (IC50 = 75 nM); p110α-H1047R (IC50 = 3 nM); p110α-E545K (IC50 = 3 nM); DNA-PK (IC50 = 1.23 μM); mTOR (Ki = 0.58 μM); Autophagy
|
|---|---|
| 体外研究 (In Vitro) |
GDC-0941 对 PI3Kα 和 PI3Kδ 以及 PI3Kα 突变体 E545-K 和 H1047-R 具有同等效力,对 PI3Kβ(10 倍)和 PI3Kγ(25 倍)表现出中等水平的选择性,对以下成员表现出较高水平的选择性: PI3K II、III 和 IV 类,包括 C2β、Vps34、DNA-PK 和 mTOR。 IC50 值分别为 46 nM、37 nM 和 28 nM。[1] GDC-0941 治疗的 IC50 为 149-944 nM,可有效减少对曲妥珠单抗敏感和不敏感的 HER2 扩增细胞的增殖。 GDC-0941 的 IC50 为 500 nM 或更低,可有效抑制具有 PIK3CA 突变的 HER2 扩增细胞的增殖以及缺乏 PTEN 且对曲妥珠单抗耐药的 HER2 扩增乳腺癌细胞的活力。 [2] GDC-0941 显着抑制 HCT116、DLD1 和 HT29 细胞的生长,GI50 值分别为 1081 nM、1070 nM 和 157 nM。 [3] GDC-0941 抑制中心母细胞数量,诱导细胞凋亡,并抑制肿瘤细胞增殖。 [4]
|
| 体内研究 (In Vivo) |
由于微粒体代谢有限,GDC-0941 的口服生物利用度为 78% [5]。在雌性 NCr 无胸腺小鼠中建立的人类 U87MG 胶质母细胞瘤异种移植物中,以 75 mg/kg/天的剂量施用 GDC-0941 可产生显着的抑制作用,肿瘤生长抑制率为 83%。 [1] 在具有 HER2 扩增、曲妥珠单抗耐药性 MDA-MB-361.1 异种移植物的小鼠中,口服 150 mg/kg/天的 GDC-0941 可显着减缓肿瘤进展并诱导有效的肿瘤细胞凋亡。 [2] GDC-0941(75 mg/kg/天)治疗两周后,PTEN+/-LKB1+/hypo 小鼠的自发性 B 细胞滤泡性淋巴瘤的肿瘤大小减少了 40%。这种肿瘤体积的减小伴随着 Akt、S6K 和 SGK(血清和糖皮质激素蛋白激酶)蛋白磷酸化的消除。 [4]
|
| 酶活实验 |
闪烁邻近分析;重组人 PI3Kα、PI3Kβ 和 PI3Kδ 在 Sf9 杆状病毒系统中与 p85α 调节亚基共表达,并使用谷胱甘肽-琼脂糖上的亲和层析纯化为 GST 融合蛋白。闪烁邻近测定法。重组人 PI3Kγ 的表达和纯化与单体 GST 融合体类似。将 GDC-0941 溶解在 DMSO 中,并注入 50 μL 20 mM Tris-HCl (pH 7.5)、4 mM MgCl2、1 mM DTT、1 μM ATP、0.125 μCi [γ-33P]-ATP 和 4% 的混合物中(v/v) 二甲基亚砜。为了启动激酶反应,将测定混合物与 PI3Kα (5 ng)、PI3Kβ (5 ng)、PI3Kδ (5 ng) 或 PI3Kγ (5 ng) 的重组 GST 融合物混合。
|
| 细胞实验 |
BT474-M1、SKBR-3、AU-565、HCC-1419、ZR75-30、JIMT-1、BT474-EEI、HCC-1954、MCF-7、CALU-3、SKOV-3 和 MKN-7 细胞是暴露于不同浓度的GDC-0941 48和72小时。 CellTiter-Glo 发光细胞活力测定用于鉴定细胞活力和增殖。通过使用蛋白质印迹,可以检查 pAkt (Ser473)、裂解的 caspase-3 和裂解的 PARP。分别使用细胞死亡检测 ELISAplus 测定法和 Caspase-Glo 3/7 测定法检测细胞凋亡和 caspase 3/7 活性。
|
| 动物实验 |
Under the skin, MCF7-neo/HER2 or MX-1 breast cancer cells are injected into female nu/nu mice. Animals are distributed into groups of 10 animals each when tumors reach a mean volume of 200 to 250 mm3. Group sizes are determined by size matching. Once a week, intravenous RP-56976, a formulation of 3% EtOH and 97% saline, is given. Pictilisib (GDC-0941), a daily oral dose of MCT (0.5% methylcellulose, 0.2% Tween-80), is administered. By directly implanting tumors from patients under the skin of NMRI nu/nu mice, the MAXF1162 HER2+/ER+/PR+ patient-derived breast cancer tumor xenograft model was created. Volume of the tumor is calculated. Throughout a study, tumor size measurements are taken twice a week.
|
| 药代性质 (ADME/PK) |
Pharmacokinetics [6]
Pharmacokinetic parameters of pictilisib were estimated for all dose cohorts and are summarized in Table 3 and Supplementary Table 1. Under fasting conditions, pictilisib was rapidly absorbed after oral administration (median Tmax of 2 hours [range 0.5-8]); this was independent of dose and was unchanged after multiple doses. Terminal plasma elimination half-life (T1/2) on day 1 ranged between 13.1 and 24.1 hours. Dose-proportional increases in exposure (Cmax and AUC0-24) was observed across the dose levels studied (Figure 1). Similar pharmacokinetic characteristics were seen on day 15. The accumulation index (AUCDay15/AUCDay1) ranged from 1.2 to 2.2, suggesting modest accumulation following multiple doses. The absorption, metabolism and excretion of pictilisib, a selective small molecule inhibitor of class 1 A phosphoinositide 3-kinase (PI3K), was characterized following a single oral administration of [14C]pictilisib in rats, dogs and humans at the target doses of 30 mg/kg, 5 mg/kg and 60 mg, respectively.Pictilisib was rapidly absorbed with Tmax less than 2 h across species. In systemic circulation, pictilisib represented the predominant total radioactivity greater than 86.6% in all species.Total pictilisib and related radioactivity was recovered from urine and faeces in rats, dogs, and human at 98%, 80% and 95%, respectively, with less than 2% excreted in urine and the rest excreted into faeces.In rat and dog, more than 40% of drug-related radioactivity was excreted into the bile suggesting biliary excretion was the major route of excretion. Unchanged pictilisib was a minor component in rat and dog bile. The major metabolite in bile was O-glucuronide of oxidation on indazole moiety (M20, 21% of the dose) in rats and an oxidative piperazinyl ring-opened metabolite M7 (10.8% of the dose) in dogs.Oxidative glutathione (GSH) conjugates (M18, M19) were novel metabolites detected in rat bile, suggesting the potential generation of reactive intermediates from pictilisib. The structure of M18 was further confirmed by NMR to be a N-hydroxylated and GSH conjugated metabolite on the moiety of the indazole ring. Xenobiotica . 2021 Jul;51(7):796-810. https://pubmed.ncbi.nlm.nih.gov/33938357/ |
| 毒性/毒理 (Toxicokinetics/TK) |
Safety and tolerability [6]
Pictilisib was well-tolerated up to 330mg (21/28 schedule); most adverse events were mild to moderate in severity with no treatment-related deaths (Table 2). At the assessed dose levels, there did not appear to be a significant difference in the toxicity profile between the 21/28 and 28/28 schedules. Treatment-related adverse events that occurred in ≥10% of patients included: nausea, diarrhea, vomiting, fatigue, dysgeusia, decreased appetite and rash. In addition to the 2 DLTs of grade 3 rash at the 450mg dose level, the third patient at this dose level experienced grade 2 rash; nonetheless, this patient received 8 months of pictilisib with concomitant use of oral antihistamines and skin emollients. Of 10 patients treated with 330mg once-daily(28/28 schedule), grade 1 or 2 rash was observed in 2 patients, and grade 3 rash (occurring after the DLT-defining window) in 2 patients; these similarly resolved with the introduction of drug holidays and supportive medications including emollients and corticosteroids. [6] Other clinically-relevant drug-related adverse events ≥grade 3 were grade 4 hyperglycemia (n=1, 130mg) and grade 3 pneumonitis (n=1, 340mg). The grade 4 hyperglycemia was transient, unaccompanied by clinically significant symptoms, signs or acidosis, and occurred in a patient with cholangiocarcinoma and previous pancreatico-duodenectomy who started the use of low-dose prednisolone 2 days prior to the event. Grade 3 pneumonitis was observed at the end of cycle 1 in a breast cancer patient previously treated with chest radiotherapy who developed grade 1 dyspnea, reduced DLCO and a ground glass appearance on HRCT; these resolved following 2 weeks of drug interruption and concomitant use of prednisolone. When pictilisib was reintroduced at 240mg, the dyspnea and HRCT changes recurred; these subsequently resolved following permanent discontinuation of pictilisib due to disease progression. DLTs and MTD [6] The MTD was exceeded at 450mg once-daily (21/28 schedule) with a DLT of grade 3 rash in 2 patients. This was a maculopapular rash covering 70-80% of the body surface area that presented approximately 2 weeks after commencement of daily pictilisib dosing and resolved spontaneously 2 weeks after treatment discontinuation. At 330mg once-daily (21/28 schedule), the grade 3 maculopapular rash observed in 1 of 7 patients had a similar temporal pattern of onset and resolution; this was also declared as a DLT. On the 28/28 schedule, no DLT was observed. |
| 参考文献 | |
| 其他信息 |
Pictilisib Bismesylate is the orally bioavailable bismesylate salt of pictilisib, a small molecule inhibitor of class I phosphatidylinositol 3 kinase (PI3K), with potential antineoplastic activity. Upon administration, pictilisib selectively binds to PI3K in an ATP-competitive manner, inhibiting the production of the secondary messenger phosphatidylinositol-3,4,5-trisphosphate (PIP3) and activation of the PI3K/Akt signaling pathway. This may result in inhibition of tumor cell growth, motility and survival in susceptible tumor cell populations. Activation of the PI3K/Akt signaling pathway is frequently associated with tumorigenesis; dysregulated PI3K/Akt signaling may contribute to tumor resistance to a variety of antineoplastic agents.
Phosphatidylinositol-3-kinase (PI3K) is an important target in cancer due to the deregulation of the PI3K/ Akt signaling pathway in a wide variety of tumors. A series of thieno[3,2-d]pyrimidine derivatives were prepared and evaluated as inhibitors of PI3 kinase p110alpha. The synthesis, biological activity, and further profiling of these compounds are described. This work resulted in the discovery of 17, GDC-0941, which is a potent, selective, orally bioavailable inhibitor of PI3K and is currently being evaluated in human clinical trials for the treatment of cancer.[1] Herceptin (trastuzumab) is the backbone of HER2-directed breast cancer therapy and benefits patients in both the adjuvant and metastatic settings. Here, we describe a mechanism of action for trastuzumab whereby antibody treatment disrupts ligand-independent HER2/HER3 interactions in HER2-amplified cells. The kinetics of dissociation parallels HER3 dephosphorylation and uncoupling from PI3K activity, leading to downregulation of proximal and distal AKT signaling, and correlates with the antiproliferative effects of trastuzumab. A selective and potent PI3K inhibitor, GDC-0941, is highly efficacious both in combination with trastuzumab and in the treatment of trastuzumab-resistant cells and tumors.[2] Background: Combined targeting of MAPK and PI3K signalling pathways may be necessary for optimal therapeutic activity in cancer. This study evaluated the MEK inhibitors AZD6244 and PD0325901, alone and in combination with the dual mTOR/PI3K inhibitor NVP-BEZ235 or the PI3K inhibitor GDC-0941, in three colorectal cancer cell lines. Methods: Growth inhibition, survival and signal transduction were measured using the Sulforhodamine B assay, clonogenicity and western blotting, respectively, in HCT116, HT29 and DLD1 cell lines. Results: All MEK/PI3K inhibitor combinations exhibited marked synergistic growth inhibition; however, GDC-0941 displayed greater synergy in combination with either MEK inhibitor. NVP-BEZ235 exhibited stronger inhibition of 4EBP1 phosphorylation, and similar inhibition of S6 and AKT phosphorylation, compared with GDC-0941. Both PD0325901 and AZD6244 inhibited ERK phosphorylation, and with MEK/PI3K inhibitor combinations inhibition of S6 phosphorylation was increased. The reduced synergy exhibited by NVP-BEZ235 in combination with MEK inhibitors, compared with GDC-0941, may be due to inhibition of mTOR, and the addition of the mTORC1/2 inhibitor KU0063794 compromised the synergy of GDC-0941:PD0325901 combinations. Conclusion: These studies confirm that dual targeting of PI3K and MEK can induce synergistic growth inhibition; however, the combination of specific PI3K inhibitors, rather than dual mTOR/PI3K inhibitors, with MEK inhibitors results in greater synergy.[3] |
| 分子式 |
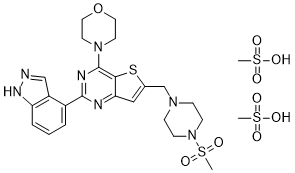
C25H35N7O9S4
|
|
|---|---|---|
| 分子量 |
705.84
|
|
| 精确质量 |
705.137
|
|
| 元素分析 |
C, 42.54; H, 5.00; N, 13.89; O, 20.40; S, 18.17
|
|
| CAS号 |
957054-33-0
|
|
| 相关CAS号 |
Pictilisib;957054-30-7
|
|
| PubChem CID |
56972143
|
|
| 外观&性状 |
White to light yellow solid powder
|
|
| 熔点 |
>280°C (dec.)
|
|
| tPSA |
270Ų
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
16
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
45
|
|
| 分子复杂度/Complexity |
924
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O=S(C)(O)=O.O=S(C)(N1CCN(CC2=CC3N=C(C4C5=C(NN=C5)C=CC=4)N=C(C=3S2)N2CCOCC2)CC1)=O
|
|
| InChi Key |
RFRIKACSFOTIMU-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C23H27N7O3S2.2CH4O3S/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19;2*1-5(2,3)4/h2-4,13-14H,5-12,15H2,1H3,(H,24,27);2*1H3,(H,2,3,4)
|
|
| 化学名 |
4-[2-(1H-indazol-4-yl)-6-[(4-methylsulfonylpiperazin-1-yl)methyl]thieno[3,2-d]pyrimidin-4-yl]morpholine;methanesulfonic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|---|
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4168 mL | 7.0838 mL | 14.1675 mL | |
| 5 mM | 0.2834 mL | 1.4168 mL | 2.8335 mL | |
| 10 mM | 0.1417 mL | 0.7084 mL | 1.4168 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。