| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|

| 体外研究 (In Vitro) |
Pitavastatin 可抑制一组培养为球体 (IC50 = 0.6-4 μM) 或单层 (IC50 = 0.4-5 μM) 的卵巢癌细胞的发育,包括那些被认为最有可能代表 HGSOC 的细胞[4]。 Ovcar-8 细胞和 Ovcar-3 细胞中刽子手 caspase-3,7 以及 caspase-8 和 caspase-9 的活性增加,表明匹伐他汀(1 微克;48 小时)会引发细胞凋亡[4]。当暴露于 1 μM 匹伐他汀 48 小时时,Ovcar-8 细胞会裂解 PARP[4]。在 TNF 刺激的人大隐静脉内皮细胞中,匹伐他汀(0.1 和 1 μM;1 小时,然后 TNF-α 孵育 6 小时)通过抑制 NF-κB 通路增强 ICAM-1 mRNA 的产生 [6]。
|
|---|---|
| 体内研究 (In Vivo) |
抑制剂匹伐他汀(59 mg/kg;口服;每天两次,持续 28 天)可显着减少肿瘤生长[4]。在饮食引起的严重高脂血症的兔模型中,匹伐他汀(0.1 mg/kg;口服;每日一次,持续 12 周)可通过上调 eNOS 和耗氧来减缓动脉粥样硬化的发展并增加 NO 生物利用度[7]。
|
| 细胞实验 |
蛋白质印迹分析[4]
细胞类型: Ovcar-8 细胞 测试浓度: 1 μM 孵育时间: > 48 小时 实验结果:诱导 PARP 裂解。 |
| 动物实验 |
Animal/Disease Models: 4 week old female NCR Nu/Nu female mice (bearing Ovcar-4 tumours)[4]
Doses: 59 mg/kg Route of Administration: po ; twice (two times) daily for 28 days Experimental Results: Caused significant tumor regression. Animal/Disease Models: Female New Zealand white rabbits (diet induced severe hyperlipidemia)[7] Doses: 0.1 mg/kg Route of Administration: po; daily for 12 weeks Experimental Results: Retarded the progression of atherosclerosis formation and improved NO bioavailability by eNOS up-regulation and decrease of O2-. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Pitavastatin peak plasma concentrations are achieved about 1 hour after oral administration. Both Cmax and AUC0-inf increased in an approximately dose-proportional manner for single pitavastatin doses from 1 mg to 24 mg once daily. The absolute bioavailability of pitavastatin oral solution is 51%. The Cmax and AUC of pitavastatin did not differ following evening or morning drug administration. In healthy volunteers receiving 4 mg pitavastatin, the percent change from baseline for LDL-C following evening dosing was slightly greater than that following morning dosing. Pitavastatin was absorbed in the small intestine but very little in the colon. Administration of pitavastatin with a high fat meal (50% fat content) decreases pitavastatin Cmax by 43% but does not significantly reduce pitavastatin AUC. Compared to other statins, pitavastatin has a relatively high bioavailability, which has been suggested to occur due to enterohepatic reabsorption in the intestine following intestinal absorption. Genetic differences in the OATP1B1 (organic-anion-transporting polypeptide 1B1) hepatic transporter encoded by the SCLCO1B1 gene (Solute Carrier Organic Anion Transporter family member 1B1) have been shown to impact pitavastatin pharmacokinetics. Evidence from pharmacogenetic studies of the c.521T>C single nucleotide polymorphism (SNP) in the gene encoding OATP1B1 (SLCO1B1) demonstrated that pitavastatin AUC was increased 3.08-fold for individuals homozygous for 521CC compared to homozygous 521TT individuals. Other statin drugs impacted by this polymorphism include [simvastatin], [pitavastatin], [atorvastatin], and [rosuvastatin]. Individuals with the 521CC genotype may be at increased risk of dose-related adverse effects including myopathy and rhabdomyolysis due to increased exposure to the drug. A mean of 15% of radioactivity of orally administered, single 32 mg 14C-labeled pitavastatin dose was excreted in urine, whereas a mean of 79% of the dose was excreted in feces within 7 days.L48616] The mean volume of distribution is approximately 148 L. Following a single dose, the apparent mean oral clearance of pitavastatin is 43.4 L/h. /MILK/ It is not known whether pitavastatin is excreted in human milk, however, it has been shown that a small amount of another drug in this class passes into human milk. Rat studies have shown that pitavastatin is excreted into breast milk. This study was addressed to understand the underlying mechanism of the substrate-dependent effect of genetic variation in SLCO1B1, which encodes OATP1B1 (organic anion transporting polypeptide) transporter, on the disposition of two OATP1B1 substrates, pravastatin and pitavastatin, in relation to their transport activities. The uptake of pravastatin, pitavastatin, and fluvastatin was measured in oocytes overexpressing SLCO1B1*1a and SLCO1B1*15 to compare the alterations of in-vitro transporting activity. After 40-mg pravastatin or 4-mg pitavastatin was administered to 11 healthy volunteers with homozygous genotypes of SLCO1B1*1a/*1a and SLCO1B1*15/*15, the pharmacokinetic parameters of pravastatin and pitavastatin were compared among participants with SLCO1B1*1a/*1a and SLCO1B1*15/*15 genotypes. The uptake of pravastatin and pitavastatin in SLCO1B1*15 overexpressing oocytes was decreased compared with that in SLCO1B1*15, but no change occurred with fluvastatin. The fold change of in-vitro intrinsic clearance (Clint) for pitavastatin in SLCO1B1*15 compared with SLCO1B1*1a was larger than that of pravastatin (P<0.0001). The clearance (Cl/F) of pitavastatin was decreased to a greater degree in participant with SLCO1B1*15/*15 compared with that of pravastatin in vivo (P<0.01), consistent with in-vitro study. As a result, Cmax and area under the plasma concentration-time curve of these nonmetabolized substrates were increased by SLCO1B1*15 variant. The greater decrease in the transport activity for pitavastatin in SLCO1B1*15 variant compared with SLCO1B1*1a was, however, associated with the greater effect on the pharmacokinetics of pitavastatin compared with pravastatin in relation to the SLCO1B1 genetic polymorphism. This study suggests that substrate dependency in the consequences of the SLCO1B1*15 variant could modulate the effect of SLCO1B1 polymorphism on the disposition of pitavastatin and pravastatin. A pharmacokinetics study was conducted in 12 Chinese volunteers following a single dose of 1 mg, 2 mg and 4 mg of pitavastatin calcium in an open-label, randomized, three-period crossover design. Plasma concentrations of pitavastatin acid and pitavastatin lactone were determined by a HPLC method. Single-nucleotide polymorphisms (SNPs) in ABCB1, ABCG2, SLCO1B1, CYP2C9 and CYP3A5 were determined by TaqMan (MGB) genotyping assay. An analysis was performed on the relationship between the aforementioned SNPs and dose-normalized (based on 1 mg) area under the plasma concentration-time curve extrapolated to infinity [AUC(0-infinity)] and peak plasma concentration (Cmax) values of the acid and lactone forms of pitavastatin. Pitavastatin exhibited linear pharmacokinetics and great inter-subject variability. Compared to CYP2C9*1/*1 carriers, CYP2C9*1/*3 carriers had higher AUC(0-infinity) and Cmax of pitavastatin acid and AUC(0-infinity) of pitavastatin lactone (P<0.05). With respect to ABCB1 G2677T/A, non-G carriers had higher Cmax and AUC(0-infinity) of pitavastatin acid, and Cmax of pitavastatin lactone compared to GT, GA or GG genotype carriers (P<0.05). Gene-dose effects of SLCO1B1 c.521T> C and g.11187G > A on pharmacokinetics of the acid and lactone forms were observed. Compared to non-SLCO1B1*17 carriers, SLCO1B1*17 carriers had higher Cmax and AUC(0-infinity) of the acid and lactone forms (P<0.05). Significant sex difference was observed for pharmacokinetics of the lactone. Female SLCO1B1 521TT subjects had higher Cmax and AUC(0-infinity) of pitavastatin lactone compared to male 521TT subjects, however, such gender difference disappeared in 521 TC and 521CC subjects. Pitavastatin pharmacokinetics was not significantly affected by ABCB1 C1236T, ABCB1C3435T, CYP3A5*3, ABCG2 c.34G > A, c.421C > A, SLCO1B1 c.388A>G, c.571T>C and c.597C>T. We conclude that CYP2C9*3, ABCB1 G2677T/A, SLCO1B1 c.521T>C, SLCO1B1 g.11187G > A, SLCO1B1*17 and gender contribute to inter-subject variability in pitavastatin pharmacokinetics. Personalized medicine should be necessary for hypercholesterolemic patients receiving pitavastatin. Pitavastatin is more than 99% protein bound in human plasma, mainly to albumin and alpha 1-acid glycoprotein, and the mean volume of distribution is approximately 148 L. Association of pitavastatin and/or its metabolites with the blood cells is minimal. For more Absorption, Distribution and Excretion (Complete) data for Pitavastatin (12 total), please visit the HSDB record page. Metabolism / Metabolites The principal route of pitavastatin metabolism is glucuronidation via liver uridine 5'-diphosphate glucuronosyltransferase (UGT) with subsequent formation of pitavastatin lactone. There is only minimal metabolism by the cytochrome P450 system. Pitavastatin is marginally metabolized by CYP2C9 and to a lesser extent by CYP2C8. The major metabolite in human plasma is the lactone, which is formed via an ester-type pitavastatin glucuronide conjugate by UGTs (UGT1A3 and UGT2B7). Pitavastatin has been studied for its effects on hepatic microsomal drug metabolism in rats, and the activities of several drug-metabolizing enzymes have been measured. No induction of the drug metabolizing enzymes (aniline hydroxylase, aminopyrine N-demethylase, 7-ethoxycoumarin O-deethylase and UDP-glucuronic acid transferase) was found in the pitavastatin group compared to the control after the multiple administrations of pitavastatin at the dosage of 1-10 mg/kg per day for 7 days. Based on several different in vitro approaches, it is concluded that CYP2C9 is the enzyme responsible for the metabolism of pitavastatin and no metabolite is present in renal and intestinal microsomes. The CYP2C9 polymorphism was not involved in the pitavastatin metabolism. No inhibitory effect in CYP-mediated metabolism was detected on the tolbutamide 4-hydroxylation (CYP2C9) and testosterone 6 beta-hydroxylation (CYP3A4) in the presence of pitavastatin. The results suggested that pitavastatin did not affect the drug-metabolizing systems. Pitavastatin is marginally metabolized by CYP2C9 and to a lesser extent by CYP2C8. The major metabolite in human plasma is the lactone which is formed via an ester-type pitavastatin glucuronide conjugate by uridine 5'-diphosphate (UDP) glucuronosyltransferase (UGT1A3 and UGT2B7). To elucidate any potential species differences, the in vitro metabolism of pitavastatin and its lactone was studied with hepatic and renal microsomes from rats, dogs, rabbits, monkeys and humans. With the addition of UDP-glucuronic acid to hepatic microsomes, pitavastatin lactone was identified as the main metabolite in several animals, including humans. Metabolic clearances of pitavastatin and its lactone in monkey hepatic microsome were much greater than in humans. M4, a metabolite of pitavastatin with a 3-dehydroxy structure, was converted to its lactone form in monkey hepatic microsomes in the presence of UDP-glucuronic acid as well as to pitavastatin. These results implied that lactonization is a common pathway for drugs such as 5-hydroxy pentanoic acid derivatives. The acid forms were metabolized to their lactone forms because of their structural characteristics. UDP-glucuronosyltransferase is the key enzyme responsible for the lactonization of pitavastatin, and overall metabolism is different compared with humans owing to the extensive oxidative metabolism of pitavastatin and its lactone in monkey. Biological Half-Life The mean plasma elimination half-life is approximately 12 hours.L48616] A mean of 15% of radioactivity of orally administered, single 32 mg (14)C-labeled pitavastatin dose was excreted in urine, whereas a mean of 79% of the dose was excreted in feces within 7 days. The mean plasma elimination half-life is approximately 12 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Pitavastatin, a hydroxymethylglutaryl-CoA (HMG-CoA) reductase inhibitor (i.e., statin), is an antilipemic agent. It is used as an adjunct to lifexstyle modifications for the management of dyslipidemias. HUMAN EXPOSURE AND TOXICITY: Pitavastatin is contraindicated for use in pregnant women or patients with active liver disease, including unexplained, persistent elevations in serum aminotransferase concentrations. Cases of myopathy and rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with HMG-CoA reductase inhibitors, including pitavastatin. These risks can occur at any dose level, but increase in a dose-dependent manner. Cases of fatal and nonfatal hepatic failure have also been reported rarely in patients receiving pitavastatin. ANIMAL STUDIES: In a 92-week carcinogenicity study in mice given pitavastatin, at the maximum tolerated dose of 75 mg/kg/day there was an absence of drug-related tumors. However, in a 92-week carcinogenicity study in rats given pitavastatin at 1, 5, 25 mg/kg/day by oral gavage, there was a significant increase in the incidence of thyroid follicular cell tumors at 25 mg/kg/day. Embryo-fetal developmental studies were conducted in pregnant rats treated with 3, 10, 30 mg/kg/day pitavastatin by oral gavage during organogenesis. No adverse effects were observed at 3 mg/kg/day. Embryo-fetal developmental studies were conducted in pregnant rabbits treated with 0.1, 0.3, 1 mg/kg/day pitavastatin by oral gavage during the period of fetal organogenesis. Maternal toxicity consisting of reduced body weight and abortion was observed at all doses tested. In perinatal/postnatal studies in pregnant rats given oral gavage doses of pitavastatin at 0.1, 0.3, 1, 3, 10, 30 mg/kg/day from organogenesis through weaning, maternal toxicity consisting of mortality at 0.3 mg/kg/day and impaired lactation at all doses contributed to the decreased survival of neonates in all dose groups. Pitavastatin had no adverse effects on male and female rat fertility at oral doses of 10 and 30 mg/kg/day, respectively. Pitavastatin was not mutagenic in the Ames test with Salmonella typhimurium and Escherichia coli with and without metabolic activation, the micronucleus test following a single administration in mice and multiple administrations in rats, the unscheduled DNA synthesis test in rats, and a Comet assay in mice. In the chromosomal aberration test, clastogenicity was observed at the highest doses tested which also elicited high levels of cytotoxicity. Hepatotoxicity Less information is available on the potential hepatotoxicity of pitavastatin in comparison to other more widely used statins. In large clinical trials, pitavastatin therapy was associated with mild, asymptomatic and usually transient serum aminotransferase elevations in approximately 1% of patients, but levels above 3 times the upper limit of normal (ULN) were infrequent and no cases of clinically apparent hepatitis were reported from the preregistration clinical trials. Since marketing of pitavastatin, however, the sponsor has received reports of jaundice, hepatitis and hepatic failure including fatal cases. However, the clinical features and typical course of the liver injury associated with pitavastatin have not been defined in the published literature. On the other hand, the other statins have all been implicated in cases of clinically apparent acute liver injury that typically arise after 1 to 6 months of therapy with either a cholestatic or hepatocellular pattern of serum enzyme elevations. Rash, fever and eosinophilia are uncommon, but some cases have been marked by autoimmune features including autoantibodies, chronic hepatitis on liver biopsy and a clinical response to corticosteroid therapy. This pattern has yet to be shown to apply to pitavastatin. Likelihood score: D (possible rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No published information exists on the use of pitavastatin during breastfeeding. It is 99% bound to plasma proteins, so amounts in milk are likely low. Because of a concern with disruption of infant lipid metabolism, the consensus is that pitavastatin should not be used during breastfeeding. However, others have argued that children homozygous for familial hypercholesterolemia are treated with statins beginning at 1 year of age, that statins have low oral bioavailability, and risks to the breastfed infant are low, especially with rosuvastatin and pravastatin. Until more data become available, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Pitavstatin is more than 99% protein bound in human plasma, mainly to albumin and alpha 1-acid glycoprotein. Interactions Pitavastatin is a substrate of organic anionic transport polypeptide (OATP) 1B1 (OATP2). Drugs that inhibit OATP1B1 (e.g., cyclosporine, erythromycin, rifampin) can increase bioavailability of pitavastatin. Concomitant use of pitavastatin (2 mg once daily) and ezetimibe (10 mg for 7 days) decreased pitavastatin peak plasma concentration and AUC by 2 and 0.2%, respectively, and increased ezetimibe peak plasma concentration and AUC by 9 and 2%, respectively. Erythromycin substantially increases pitavastatin exposure.1 Following concomitant use of pitavastatin (4 mg as a single dose on day 4) and erythromycin (500 mg 4 times daily for 6 days), pitavastatin peak plasma concentration and AUC were increased by 3.6- and 2.8-fold, respectively; such effects were considered clinically important. The interaction between pitavastatin and erythromycin probably resulted partly from erythromycin-induced inhibition of organic anionic transport polypeptide (OATP)1B1-mediated hepatic uptake of pitavastatin. If used concomitantly with erythromycin, dosage of pitavastatin should not exceed 1 mg once daily. Concomitant use of pitavastatin (4 mg once daily on days 1-5 and 11-15) and extended-release diltiazem hydrochloride (240 mg on days 6-15) increased pitavastatin peak plasma concentration and AUC by 15 and 10%, respectively, and decreased diltiazem peak plasma concentration and AUC by 7 and 2%, respectively. For more Interactions (Complete) data for Pitavastatin (20 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Hydroxymethylglutaryl-CoA Reductase Inhibitors Livalo is indicated as an adjunctive therapy to diet to reduce elevated total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), apolipoprotein B (Apo B), triglycerides (TG), and to increase HDL-C in adult patients with primary hyperlipidemia or mixed dyslipidemia. /Included in US product label/ The American College of Cardiology (ACC)/American Heart Association (AHA) cholesterol management guideline recommends statins as first-line therapy for prevention of atherosclerotic cardiovascular disease (ASCVD) in adults. Pitavastatin may be used for primary or secondary prevention in adults when moderate-intensity statin therapy is indicated. /NOT included in US product label/ /EXPL THER/ Pitavastatin classically functions as a blood cholesterol-lowering drug. Previously, it was discovered with antiglioma stem cell properties through drug screening. However, whether it can be used for liver cancer cell therapy has never been reported. In this study, the cell viability and colony formation assay were utilized to analyze the cytotoxicity of pitavastatin on liver cancer cells. The cell cycle alteration was checked after pitavastatin treatment. Apoptosis-related protein expression and the effect of caspase inhibitor were also checked. The in vivo inhibitory effect of pitavastatin on the growth of liver tumor was also tested. It was found that pitavastatin inhibited growth and colony formation of liver cancer Huh-7 cells and SMMC7721 cells. It induced arrest of liver cancer cells at the G1 phase. Increased proportion of sub-G1 cells was observed after pitavastatin treatment. Pitavastatin promoted caspase-9 cleavage and caspase-3 cleavage in liver cancer cells. Caspase inhibitor Z-VAD-FMK reversed the cleavage of cytotoxic effect of pitavastatin. Moreover, pitavastatin decreased the tumor growth and improved the survival of tumor-bearing mice. This study suggested the antiliver cancer effect of the old drug pitavastatin. It may be developed as a drug for liver cancer therapy. Drug Warnings Increases in serum aminotransferase (i.e., AST [SGOT], ALT [SGPT]) concentrations have been reported in patients receiving statins, including pitavastatin. These increases usually were transient and resolved or improved with continued therapy or after temporary interruption of therapy. In phase 2, placebo-controlled studies, increases in serum ALT concentrations exceeding 3 times the upper limit of normal occurred in 0.5% of patients receiving pitavastatin 4 mg daily. Cases of fatal and nonfatal hepatic failure have been reported rarely in patients receiving statins, including pitavastatin, during postmarketing surveillance. Immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, has been reported rarely in patients receiving statins. Immune-mediated necrotizing myopathy is characterized by proximal muscle weakness and elevated creatine kinase (CK, creatine phosphokinase, CPK) concentrations that persist despite discontinuance of statin therapy, necrotizing myopathy without substantial inflammation, and improvement following therapy with immunosuppressive agents. Pitavastatin should be used with caution in patients with predisposing factors for myopathy (e.g., advanced age [older than 65 years of age], renal impairment, inadequately-treated hypothyroidism) and in patients receiving concomitant therapy with certain antilipemic agents (i.e., fibric acid derivatives, antilipemic dosages of niacin). Cases of myopathy and rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with HMG-CoA reductase inhibitors, including Livalo. These risks can occur at any dose level, but increase in a dose-dependent manner. Pitavastatin is distributed into milk in rats. It is not known whether pitavastatin is distributed into human milk; however, a small amount of another statin is distributed into human milk. Because of the potential for serious adverse reactions from pitavastatin in nursing infants, the drug is contraindicated in nursing women. Women who require pitavastatin therapy should be advised not to breast-feed their infants or advised to discontinue pitavastatin. For more Drug Warnings (Complete) data for Pitavastatin (26 total), please visit the HSDB record page. Pharmacodynamics Pitavastatin is an oral antilipemic agent which inhibits HMG-CoA reductase. It is used to lower total cholesterol, low density lipoprotein-cholesterol (LDL-C), apolipoprotein B (apoB), non-high density lipoprotein-cholesterol (non-HDL-C), and trigleride (TG) plasma concentrations while increasing HDL-C concentrations. High LDL-C, low HDL-C and high TG concentrations in the plasma are associated with increased risk of atherosclerosis and cardiovascular disease. The total cholesterol to HDL-C ratio is a strong predictor of coronary artery disease and high ratios are associated with higher risk of disease. Increased levels of HDL-C are associated with lower cardiovascular risk. By decreasing LDL-C and TG and increasing HDL-C, rosuvastatin reduces the risk of cardiovascular morbidity and mortality. Elevated cholesterol levels, and in particular, elevated low-density lipoprotein (LDL) levels, are an important risk factor for the development of CVD. Use of statins to target and reduce LDL levels has been shown in a number of landmark studies to significantly reduce the risk of development of CVD and all-cause mortality. Statins are considered a cost-effective treatment option for CVD due to their evidence of reducing all-cause mortality including fatal and non-fatal CVD as well as the need for surgical revascularization or angioplasty following a heart attack. Evidence has shown that even for low-risk individuals (with <10% risk of a major vascular event occurring within 5 years) statins cause a 20%-22% relative reduction in major cardiovascular events (heart attack, stroke, coronary revascularization, and coronary death) for every 1 mmol/L reduction in LDL without any significant side effects or risks. **Skeletal Muscle Effects** Pitavastatin may cause myopathy (muscle pain, tenderness, or weakness with creatine kinase (CK) above ten times the upper limit of normal) and rhabdomyolysis (with or without acute renal failure secondary to myoglobinuria). Rare fatalities have occurred as a result of rhabdomyolysis with statin use, including pitavastatin. Predisposing factors for myopathy include advanced age (≥65 years), female gender, uncontrolled hypothyroidism, and renal impairment. In most cases, muscle symptoms and CK increases resolved when treatment was promptly discontinued. As dosages of pitavastatin greater than 4mg per day were associated with an increased risk of severe myopathy, the product monograph recommends a maximum daily dose of 4mg once daily. The risk of myopathy during treatment with pitavstatin may be increased with concurrent administration of interacting drugs such as [fenofibrate], [niacin], [gemfibrozil], and [cyclosporine]. Cases of myopathy, including rhabdomyolysis, have been reported with HMG-CoA reductase inhibitors coadministered with [colchicine], and caution should therefore be exercised when prescribing these two medications together. Real-world data from observational studies has suggested that 10-15% of people taking statins may experience muscle aches at some point during treatment. **Hepatic Dysfunction** Increases in serum transaminases have been reported with pitavastatin. In most cases, the elevations were transient and either resolved or improved on continued therapy or after a brief interruption in therapy. There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including pitavastatin. Patients who consume substantial quantities of alcohol and/or have a history of liver disease may be at increased risk for hepatic injury. **Increases in HbA1c and Fasting Serum Glucose Levels** Increases in HbA1c and fasting serum glucose levels have been reported with statins, including pitavastatin. Optimize lifestyle measures, including regular exercise, maintaining a healthy body weight, and making healthy food choices. An in vitro study found that [atorvastatin], [pravastatin], [rosuvastatin], and [pitavastatin] exhibited a dose-dependent cytotoxic effect on human pancreas islet β cells, with reductions in cell viability of 32, 41, 34 and 29%, respectively, versus control. Moreover, insulin secretion rates were decreased by 34, 30, 27 and 19%, respectively, relative to control. |
| 分子式 |
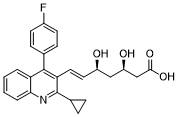
C25H24FNO4
|
|---|---|
| 分子量 |
421.46
|
| 精确质量 |
421.168
|
| CAS号 |
147511-69-1
|
| 相关CAS号 |
Pitavastatin Calcium;147526-32-7;Pitavastatin-d4;2070009-71-9;Pitavastatin-d5 sodium;Pitavastatin sodium;574705-92-3
|
| PubChem CID |
5282452
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
692.0±55.0 °C at 760 mmHg
|
| 闪点 |
372.3±31.5 °C
|
| 蒸汽压 |
0.0±2.3 mmHg at 25°C
|
| 折射率 |
1.680
|
| LogP |
3.45
|
| tPSA |
90.65
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
31
|
| 分子复杂度/Complexity |
631
|
| 定义原子立体中心数目 |
2
|
| SMILES |
C1CC1C2=NC3=CC=CC=C3C(=C2/C=C/[C@H](C[C@H](CC(=O)O)O)O)C4=CC=C(C=C4)F
|
| InChi Key |
VGYFMXBACGZSIL-MCBHFWOFSA-N
|
| InChi Code |
InChI=1S/C25H24FNO4/c26-17-9-7-15(8-10-17)24-20-3-1-2-4-22(20)27-25(16-5-6-16)21(24)12-11-18(28)13-19(29)14-23(30)31/h1-4,7-12,16,18-19,28-29H,5-6,13-14H2,(H,30,31)/b12-11+/t18-,19-/m1/s1
|
| 化学名 |
(E,3R,5S)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl]-3,5-dihydroxyhept-6-enoic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~237.27 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.93 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.93 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3727 mL | 11.8635 mL | 23.7270 mL | |
| 5 mM | 0.4745 mL | 2.3727 mL | 4.7454 mL | |
| 10 mM | 0.2373 mL | 1.1864 mL | 2.3727 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Effect of Pitavastatin on Bone
CTID: NCT06359353
Phase: Phase 4 Status: Completed
Date: 2024-04-11