| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
体外活性:泊沙康唑具有有效的杀锥虫活性。胺碘酮与泊沙康唑具有协同作用。泊沙康唑还会影响和破坏克氏锥虫的 Ca2+ 稳态。泊沙康唑阻断麦角甾醇的生物合成,麦角甾醇对于寄生虫的生存至关重要。泊沙康唑对上鞭毛体(细胞外)阶段的增殖具有明显的剂量依赖性影响,最小抑制浓度为 20 nM,IC50 为 14 nM。泊沙康唑针对临床相关的细胞内无鞭毛体形式的寄生虫更有效。泊沙康唑的最小抑制浓度和 IC50 值为 3 nM 和 0.25 nM。泊沙康唑对念珠菌和曲霉属的分离株具有活性。对氟康唑、伏立康唑和两性霉素 B 表现出耐药性,并且比其他三唑类药物对接合菌的活性更强。细胞测定:寄生虫的上鞭毛体形式在肝输注胰蛋白胨培养基中培养,补充有 10% 新生小牛血清,28°C 强烈(120 rpm)搅拌。以 2 × 106 上鞭毛体/mL 的细胞密度开始培养,并以 0.5−1.0 × 107 上鞭毛体/mL 的细胞密度添加泊沙康唑。细胞密度通过使用电子粒子计数器以及通过血细胞计数器直接计数来测量。使用光学显微镜,通过台盼蓝排除法追踪细胞活力。无鞭毛体在 Vero 细胞中培养,维持在补充有 1% 胎牛血清的基本必需培养基中,在潮湿气氛(95% 空气−5% CO2)中于 37 °C 下进行。每个细胞用 10 个组织培养来源的锥鞭毛体感染细胞 2 小时,然后用磷酸盐缓冲盐水 (PBS) 洗涤 3 次以去除未粘附的寄生虫。添加含有和不含泊沙康唑的新鲜培养基,将细胞孵育96小时,并在48小时更换培养基。使用光学显微镜直接测定受感染细胞的百分比和每个细胞的寄生虫数量,并对结果进行统计分析。 IC50 值使用 GraFit 程序通过非线性回归计算。计算分数抑制浓度(FIC)。使用 Fura-2 通过荧光法测定对照和药物处理的细胞外上鞭毛体中的细胞质游离 Ca2+ 浓度。使用时间扫描共聚焦显微镜监测感染克氏锥虫无鞭毛体的单个 Vero 细胞的亚细胞 Ca2+ 水平和线粒体膜电位。简而言之,将被克氏锥虫无鞭毛体重度感染(72 小时)的 Vero 细胞铺在 22 × 40 mm 玻璃盖玻片(0.15 mm 厚)上,并与 10 μM 细胞渗透性 Rhod-2 和 10 μg/mL Rhodamine-123 同时孵育在培养基中 37°C 培养 50 分钟,然后用林格氏溶液(含或不含胺碘酮)洗涤并孵育。在使用的条件下,Rhod-2 的荧光主要来自细胞内富含 Ca2+ 的区室,如线粒体,因为它对 Ca2+ 的低亲和力限制了其在 Vero 细胞或无鞭毛体的贫 Ca2+ 细胞质中的荧光。 Rhodamine-123 是一种线粒体特异性阳离子染料,严格根据膜电位分布在线粒体内膜上。
|
|---|---|
| 体内研究 (In Vivo) |
单独用胺碘酮治疗受感染的动物可以减少寄生虫血症,增加感染后 60 天的存活率(未治疗的对照组为 0%,而胺碘酮治疗的动物为 40%),并且当与泊沙康唑联合使用时,可以延缓寄生虫血症的发展。与空腹状态下单独服用泊沙康唑相比,泊沙康唑和 Boost Plus 联合服用会增加药物暴露量。食物,特别是脂肪含量高的膳食,可显着增加泊沙康唑的生物利用度。当与高脂肪和脱脂膳食一起食用时,泊沙康唑的全身暴露量分别增加 4 倍和 2.6 倍。泊沙康唑和胺碘酮可能构成有效的抗结核杆菌。 cruzi疗法副作用低。每日两次剂量≥15毫克/公斤体重时,泊沙康唑可延长小鼠的存活时间并减轻组织负担。
|
| 细胞实验 |
该寄生虫的上鞭毛体形式在添加了 10% 新鲜小牛血清的肝脏灌注胰蛋白胨培养基 12 中生长,温度为 28°C,剧烈(120 rpm)搅拌。在以 2×106 上鞭毛体 mL-1 的密度开始培养后,将药物以 0.5−1.0 ×107 上鞭毛体 mL-1 的细胞密度添加到培养物中。血细胞计数器直接计数和电子粒子计数均用于测量细胞密度。台盼蓝排除用于在光学显微镜下测量细胞活力。如前所述,无鞭毛体在 Vero 细胞中培养,该细胞保存在补充有 1% 胎牛血清的基本必需培养基中,在 37°C 的湿润气氛(95% 空气−5% CO2)下进行。每个细胞用 10 个来自组织培养的锥鞭毛体感染两小时后,通过三轮磷酸盐缓冲盐水 (PBS) 洗涤消除非粘附寄生虫。细胞培养 96 小时,每 48 小时更换一次培养基,添加含药物和不含药物的新鲜培养基。使用光学显微镜,可以直接测量受感染细胞的百分比和每个细胞的寄生虫数量。然后如前所述对数据进行统计分析。使用 GraFit 程序,使用非线性回归来计算 IC50 值。再次使用 Fura-2,荧光技术用于测定对照和药物处理的细胞外上鞭毛体中的细胞质游离 Ca2+ 浓度(参见前面的描述)。使用时间扫描共聚焦显微镜测量感染克氏锥虫无鞭毛体的单个 Vero 细胞的亚细胞 Ca2+ 水平和线粒体膜电位;该技术在其他地方有详细介绍。简而言之,Vero 细胞经历了 72 小时的重度 T 感染。将 Cruzi 无鞭毛体铺在尺寸为 22 x 40 毫米、厚度为 0.15 毫米的玻璃盖玻片上。然后将它们在含有 10 μM 细胞渗透性 Rhod-2 和 10 μg/mL Rhodamine-123 的培养基中于 37°C 孵育 50 分钟。此后,将它们冲洗并用林格氏溶液(含或不含胺碘酮)孵育。 Rhod-2 对 Ca2+ 的低亲和力限制了其在 Vero 细胞或无鞭毛体缺乏 Ca2+ 的细胞质中发出的荧光,因此在使用的条件下,其荧光主要源自细胞内富含 Ca2+ 的区室,例如线粒体。一种名为罗丹明-123 的阳离子染料是线粒体所特有的,它严格按照膜电位分布在内膜上。
|
| 动物实验 |
The murine model of acute Chagas disease is used for in vivo studies. Female NMRI-IVIC mice (20–25 g) are infected with 105 or 103 bloodstream trypomastigotes, and drug treatment is initiated 24 hours later. For 30 days in a row, treatments consist of 30 doses of posaconazole (20 mg/kg/d) or 15 doses of amiodarone (5 mg/kg every other day).Positive controls are given the anti-T. cruzi drug nifurtimox at a dose of 50 mg/kg/d for 30 days, whereas negative controls, or animals that are not given any treatment, are given just the vehicle. Every day, survival is monitored, and every week, parasitemia is assessed through direct microscopic inspection. After 60 days of observation following infection, parasitological cures are assessed using a combination of blood PCR tests, xenodiagnosis, and hemoculture.
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Posaconazole is absorbed with a median Tmax of approximately 3 to 5 hours. The excreted metabolites in urine and feces account for ~17% of the administered radiolabeled dose. 1774 L 32 L/hr 51 L/hr [Single-Dose Suspension Administration of 200 mg, fasted] 21 L/hr [Single-Dose Suspension Administration of 200 mg, nonfat meal] 14 L/hr [Single-Dose Suspension Administration of 200 mg, high fat meal] 91 L/hr [Single-Dose Suspension Administration of 400 mg, fasted] 43 L/hr [Single-Dose Suspension Administration of 400 mg with liquid nutritional supplement (14 g fat)] Kinetics and protein binding following oral posaconazole dosing were performed in neutropenic infected mice. Peak levels and AUC from 0 hr to infinity values were nonlinear over the 16-fold dose range studied. Serum drug elimination half-life ranged from 12.0 to 17.7 hr The suspension formulation of posaconazole was associated with enhanced systemic exposure and increased relative bioavailability compared with the tablet. Food substantially enhanced the rate and extent of posaconazole absorption in healthy subjects. A total of 103 healthy adults were enrolled in two phase I trials. Each study had a double-blind, placebo-controlled, parallel-group design with a rising single-dose (RSD) or rising multiple-dose (RMD) scheme. In the RSD study, subjects received single doses of posaconazole oral tablets (50 to 1200 mg) or placebo. In the RMD study, subjects received posaconazole oral tablets (50 to 400 mg) or placebo twice daily for 14 days. By using model-independent methods, the area under the plasma concentration-time curve and the maximum concentration in plasma were determined and used to assess dose proportionality. In the RSD study, the levels of posaconazole in plasma increased proportionally between the 50- and 800-mg dose range, with saturation of absorption occurring above 800 mg. Dose proportionality was also observed in the RMD study. In both studies, the apparent volume of distribution was large (range, 343 to 1341 liters) and the terminal-phase half-life was long (range, 25 to 31 hr). Subjects fasted 12 hours before and 48 hours after the administration of posaconazole oral suspension (800 mg) given as a single dose (regimen A), 400 mg every 12 hours (regimen B) or 200 mg every 6 hours (regimen C). Plasma posaconazole concentrations were determined for 48 hours after the initial dose and subjects completed a 1-week washout period between treatment regimens. A one-compartment oral model with first-order rate of absorption and first-order rate of elimination was fitted to the plasma concentration-time data. Differences in exposure were investigated by allowing the bioavailability fraction to vary among regimens. A total of 18 healthy men were enrolled in and completed the study. : Posaconazole relative bioavailability was estimated to be significantly different among regimens (p < 0.0001) and increased with the number of doses, such that regimen B/regimen A = 1.98 +/- 0.35, representing a 98% increase, and regimen C/regimen A = 3.20 +/- 0.69, or a 220% increase. With use of the one-compartment model, the population steady-state values for area under the concentration-time curve over 24 hours were predicted to be 3900, 7700 and 12 400 microg.h/L, with average plasma concentrations of 162, 320 and 517 microg/L for regimens A, B and C, respectively. These data suggest that divided daily dose administration (every 12 or 6 hours) significantly increases posaconazole exposure under fasted conditions. For more Absorption, Distribution and Excretion (Complete) data for POSACONAZOLE (6 total), please visit the HSDB record page. Metabolism / Metabolites Posaconazole primarily circulates as the parent compound in plasma. Of the circulating metabolites, the majority are glucuronide conjugates formed via UDP glucuronidation (phase 2 enzymes). Posaconazole does not have any major circulating oxidative (CYP450 mediated) metabolites. The excreted metabolites in urine and feces account for ~17% of the administered radiolabeled dose. Biological Half-Life Posaconazole is eliminated with a mean half-life (t½) of 35 hours (range 20 to 66 hours). The i.v. terminal-phase half-lives were 7 hr in mice and rats, 15 hr in dogs, and 23 hr in monkeys. In rabbits, the oral half-life was 9 hr. Kinetics and protein binding following oral posaconazole dosing were performed in neutropenic infected mice. Peak levels and AUC from 0 hr to infinity values were nonlinear over the 16-fold dose range studied. Serum drug elimination half-life ranged from 12.0 to 17.7 hr |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Transient elevations in serum aminotransferase levels occur in 2% to 12% of patients on posaconazole. These elevations are usually mild, asymptomatic and self-limited and rarely require discontinuation of the medication. Clinically apparent hepatotoxicity is very rare. Instances of jaundice and hepatitis during posaconazole therapy are mentioned in the product label, but little information was provided on clinical details. Likelihood score: E* (unproved but suspected cause of clinically apparent liver injury). Protein Binding Posaconazole is highly protein bound (>98%), predominantly to albumin. Interactions Drug interactions mediated by various CYP450 are common with the currently available triazole antifungals, however ... posaconazole may have an improved and more narrow drug interaction profile (CYP3A4 only) compared with other triazoles. This study evaluated the potential for a pH-dependent pharmacokinetic interaction between posaconazole and an antacid (Mylanta), under fasting and nonfasting conditions. Twelve men completed this randomized, four-period crossover, single-dose study. Subjects received 200 mg of posaconazole following a 10-h fast, with 20 ml of Mylanta and a 10-h fast, with 20 ml of Mylanta and a high-fat breakfast, and with a high-fat breakfast alone. Antacid coadministration had no statistically significant effects on posaconazole bioavailability under fasting or nonfasting conditions. In the fasting state, antacid slightly increased the relative oral bioavailability of posaconazole by 15% (P = 0.296); in the nonfasting state, antacid decreased the relative bioavailability of posaconazole by 12% (P = 0.352). Food increased the relative oral bioavailability of posaconazole by 400% (P = 0.001). In conclusion, the effect of antacid on posaconazole exposure in the fasting or nonfasting state was small and is not considered clinically significant. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Mesh Heading: Antibiotics, antifungals, trypanocidal agents MEDICATION: Antifungal; Orally activated triazole antifungal The pharmacokinetics of posaconazole oral suspension in neutropenic patients undergoing high-dose chemotherapy and stem cell transplantation were evaluated, and the association of plasma posaconazole exposure with the presence and severity of oral mucositis was explored in this nonrandomized, open-label, parallel-group, multiple-dose pharmacokinetic study. Thirty patients were enrolled and received one of three regimens (group I, 200 mg once daily; group II, 400 mg once daily; group III, 200 mg four times daily) for the duration of neutropenia. The mean total exposure for day 1, as shown by the area under the concentration-time curve from 0 to 24 h (AUC(0-24)), was 1.96 mg . h/liter in group I and was 51% higher in group II and in group III. Increases in AUC(0-24) and maximum plasma concentration (C(max)) in groups II and III were dose related. The AUC(0-24) and C(max) values on day 1 were similar between groups II and III. There was interpatient variability of up to 68% in the pharmacokinetic values for our study population. Steady state was attained by days 5 to 6. Average steady-state plasma posaconazole trough values were 192, 219, and 414 ng/ml in groups I, II, and III, respectively. The AUC(0-24) and apparent oral clearance increased by increasing dose and dosing frequency. Mucositis appeared to reduce exposure but did not significantly affect mean total posaconazole exposure (AUC and C(max)) at steady state (P = 0.1483). Moreover, this reduction could be overcome by increasing the total dose and dosing frequency. Posaconazole was safe and well tolerated. /EXPTL:/ ... Posaconazole has demonstrated strong antifungal efficacy in Phase II and III clinical trials in immunocompromised patients with oropharyngeal and esophageal candidiasis. Posaconazole also showed promising efficacy as salvage therapy in a large Phase II study including 330 patients with invasive fungal infections intolerant to or refractory to standard therapies. ... Drug Warnings Invasive fungal infections are found most frequently in immunosuppressed and critically ill hospitalized patients. Antifungal therapy is often required for long periods. Safety data from the clinical development program of the triazole antifungal agent, posaconazole, were analyzed. A total of 428 patients with refractory invasive fungal infections (n = 362) or febrile neutropenia (n = 66) received posaconazole in 2 phase II/III open-label clinical trials. Also, 109 of these patients received posaconazole therapy for > or = 6 months. Incidences of treatment-emergent, treatment-related, and serious adverse events and abnormal laboratory parameters were recorded during these studies. Treatment-emergent, treatment-related adverse events were reported in 38% of the overall patient population. The most common treatment-related adverse events were nausea (8%) and vomiting (6%). Treatment-related serious adverse events occurred in 8% of patients. Low rates of treatment-related corrected QT interval and/or QT interval prolongation (1%) and elevation of hepatic enzymes (2%) were reported as adverse events. Treatment-emergent, treatment-related adverse events occurred at similar rates in patients who received posaconazole therapy for < 6 months and > or = 6 months. Prolonged posaconazole treatment was associated with a generally favorable safety profile in seriously ill patients with refractory invasive fungal infections. Long-term therapy did not increase the risk of any individual adverse event, and no unique adverse event was observed with longer exposure to posaconazole. The pharmacokinetic profiles, safety, and efficacies of different dosing schedules of posaconazole oral suspension in patients with possible, probable, and proven refractory invasive fungal infection (rIFI) or febrile neutropenia (FN) were evaluated in a multicenter, open-label, parallel-group study. Sixty-six patients with FN and 32 patients with rIFI were randomly assigned to one of three posaconazole regimens: 200 mg four times a day (q.i.d.) for nine doses, followed by 400 mg twice a day (b.i.d.); 400 mg q.i.d. for nine doses, followed by 600 mg b.i.d.; or 800 mg b.i.d. for five doses, followed by 800 mg once a day (q.d.). Therapy was continued for up to 6 months in patients with rIFI or until neutrophil recovery occurred in patients with FN. The 400-mg-b.i.d. dose provided the highest overall mean exposure, with 135% (P = 0.0004) and 182% (P < 0.0001) greater exposure than the 600-mg-b.i.d. and 800-mg-q.d. doses, respectively. However, exposure in allogeneic bone marrow transplant (BMT) recipients (n = 12) was 52% lower than in non-BMT patients. Treatment-related adverse events (occurring in 24% of patients) were mostly gastrointestinal in nature. Twenty-four percent of patients had adverse events leading to premature discontinuation (none were treatment related). In efficacy-evaluable patients, successful clinical response was observed in 43% with rIFI (56% of patients receiving 400 mg b.i.d., 17% receiving 600 mg b.i.d., and 50% receiving 800 mg q.d.) and 77% with FN (74% receiving 400 mg b.i.d., 78% receiving 600 mg b.i.d., and 81% receiving 800 mg q.d.). Posaconazole is well tolerated and absorbed. Divided doses of 800 mg (400 mg b.i.d.) provide the greatest posaconazole exposure. The authors evaluated the pharmacokinetics and safety of posaconazole in healthy subjects and in those with mild (CL(CR) = 50-80 mL/min), moderate (CL(CR) = 20-49 mL/min), and severe chronic renal disease (CL(CR) <20 mL/min; receiving outpatient hemodialysis) (n = 6/group). Subjects received one 400-mg dose of posaconazole oral suspension with a standardized high-fat breakfast. For hemodialysis-dependent subjects, this dose was given on a nonhemodialysis day, and a second 400-mg dose was given 6 hours before hemodialysis. ...There was no correlation between posaconazole pharmacokinetics and mild to moderate renal disease ...Furthermore, the difference in the predialyzed and postdialyzed posaconazole concentrations was only approximately 3%, supporting that posaconazole was not removed by hemodialysis. ... Pharmacodynamics Posaconazole is an antifungal agent structurally related to itraconazole. It is a drug derived from itraconzaole through the replacement of the chlorine substituents with flourine in the phenyl ring, as well as hydroxylation of the triazolone side chain. These modifications enhance the potency and spectrum of activity of the drug. Posaconazole can be either fungicial or fungistatic in action. |
| 分子式 |
C37H42F2N8O4
|
|---|---|
| 分子量 |
700.78
|
| 精确质量 |
700.329
|
| 元素分析 |
C, 63.41; H, 6.04; F, 5.42; N, 15.99; O, 9.13
|
| CAS号 |
171228-49-2
|
| 相关CAS号 |
Posaconazole-d5;1217785-83-5;Posaconazole-d4;1133712-26-1;Posaconazole hydrate;1198769-38-8
|
| PubChem CID |
468595
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
850.7±75.0 °C at 760 mmHg
|
| 熔点 |
170-1720C
|
| 闪点 |
468.3±37.1 °C
|
| 蒸汽压 |
0.0±0.3 mmHg at 25°C
|
| 折射率 |
1.658
|
| LogP |
2.25
|
| tPSA |
115.7
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
12
|
| 重原子数目 |
51
|
| 分子复杂度/Complexity |
1170
|
| 定义原子立体中心数目 |
4
|
| SMILES |
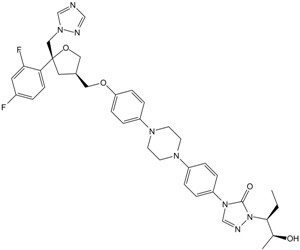
FC1=CC=C([C@@]2(CN3C=NC=N3)C[C@H](COC4=CC=C(N5CCN(C6=CC=C(N7C=NN([C@@H](CC)[C@H](C)O)C7=O)C=C6)CC5)C=C4)CO2)C(F)=C1
|
| InChi Key |
RAGOYPUPXAKGKH-XAKZXMRKSA-N
|
| InChi Code |
InChI=1S/C37H42F2N8O4/c1-3-35(26(2)48)47-36(49)46(25-42-47)31-7-5-29(6-8-31)43-14-16-44(17-15-43)30-9-11-32(12-10-30)50-20-27-19-37(51-21-27,22-45-24-40-23-41-45)33-13-4-28(38)18-34(33)39/h4-13,18,23-27,35,48H,3,14-17,19-22H2,1-2H3/t26-,27+,35-,37-/m0/s1
|
| 化学名 |
4-[4-[4-[4-[[(3R,5R)-5-(2,4-difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl)oxolan-3-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-2-[(2S,3S)-2-hydroxypentan-3-yl]-1,2,4-triazol-3-one
|
| 别名 |
Posaconazole; Noxafil; SCH-56592; Schering 56592; Sch 56592; Schering 56592;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 18.75~100 mg/mL ( 26.76~142.69 mM )
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1.88 mg/mL (2.68 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液。
例如,若需制备1 mL的工作液,可将100 μL 18.8 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1.88 mg/mL (2.68 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 18.8mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1.88 mg/mL (2.68 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 1.88 mg/mL (2.68 mM) 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4270 mL | 7.1349 mL | 14.2698 mL | |
| 5 mM | 0.2854 mL | 1.4270 mL | 2.8540 mL | |
| 10 mM | 0.1427 mL | 0.7135 mL | 1.4270 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Posaconazole Prophylaxis During ATG Treatment for hMDS/AA Patients
CTID: NCT03318159
Phase: Phase 2 Status: Completed
Date: 2024-04-17