| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
VEGFR2 (IC50 = 12 nM); PDGFR (IC50 = 2 nM); mTOR (IC50 = 10 nM); DNK-PK (IC50 = 60 nM); Src (IC50 = 14 nM)
|
|---|---|
| 体外研究 (In Vitro) |
PP121 通过直接抑制两种胶质母细胞瘤细胞系 U87 和 LN229 中的 PI3K/mTOR 来阻断 PI3K 通路。 PP121 可有效抑制 PI3-K 通路成员 PIK3CA、PTEN 或 RAS 发生突变的多种肿瘤细胞系的增殖。大多数肿瘤细胞被 PP121 置于 G0G1 停滞状态。 Src在细胞中被PP121直接抑制,这也逆转了Src的生化和形态效应。在体外,PP121 有效抑制 Ret 激酶结构域 (IC50<1 nM)。当 PI3-K 和 MAPK 途径被 VEGF 激活时,PP121 会有效阻断它们。 PP121 在低纳摩尔浓度下抑制 VEGFR2 自磷酸化,这一事实支持了 PP121 直接靶向细胞中的 VEGFR2。在表达 Bcr-Abl 的 K562 细胞和 BaF3 细胞中,PP121 抑制 Bcr-Abl 诱导的酪氨酸磷酸化[1]。
|
| 体内研究 (In Vivo) |
口服PP121可显着抑制Eca-109异种移植物的生长。 PP121 或载体治疗对小鼠的体重没有明显影响。在异种移植肿瘤中,口服 PP121 显着降低 Akt-mTOR 和 NFkB 激活。 PP121 的施用会抑制 p-IKKa/b 和 p-Akt Ser 473[2]。
|
| 酶活实验 |
在 10 µM ATP、2.5 µCi γ 存在的情况下,将纯化的激酶结构域与浓度范围为 50-0.001 M 的 2 或 4 倍稀释的抑制剂 (PP121) 一起孵育,或与载体 (0.1% DMSO) 一起孵育- 32P-ATP 和底物。根据底物的不同,通过点滴到硝酸纤维素膜或磷酸纤维素膜上来停止反应。然后将该膜清洗 5-6 次以除去未与其结合的放射性物质,然后将其干燥。 Prism 软件用于通过磷光成像量化转移的放射性,并通过将数据拟合到 S 形剂量反应曲线来生成 IC50 值 [1]。
|
| 细胞实验 |
PP121 以 4 倍稀释 (10 µM - 0.040 µM) 或载体 (0.1% DMSO) 应用于 96 孔板中生长的细胞。 72 小时后,将细胞暴露于刃天青钠盐 (22 µM),并测量荧光。计算 IC50 值。非贴壁细胞以低密度(3-5% 汇合)铺板,并用药物 (2.5 µM) 或载体 (0.1% DMSO) 处理,用于涉及单细胞计数的增殖测定。每天,细胞用台盼蓝稀释并使用血细胞计数器进行计数[1]。
|
| 动物实验 |
Mice: Eca-109 cells are injected into the axillary regions of nude mice (5×106 cells/mouse). When the tumor volumes reach around 200 mm3, the mice are randomly separated to three groups: Untreated control, PP121 (30 mg/kg) and vehicle (10% 1-methyl-2-pyrrolidinone and 90% PEG 300) group. Tumor volumes and the mice body weights are measured every 10 d[2].
|
| 参考文献 |
|
| 其他信息 |
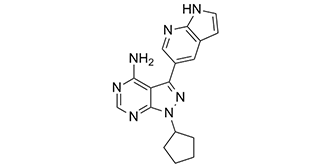
PP121 is a pyrazolopyrimidine that is 1H-pyrazolo[3,4-d]pyrimidine which is substituted by a cyclopentyl, 1H-pyrrolo[2,3-b]pyridin-5-yl, and amino groups at positions 1, 3 and 4, respectively. It is a dual inhibitor of tyrosine and phosphoinositide kinases and exhibits anti-cancer properties. It has a role as an EC 2.7.1.137 (phosphatidylinositol 3-kinase) inhibitor, a tyrosine kinase inhibitor and an antineoplastic agent. It is a pyrazolopyrimidine, a pyrrolopyridine, a member of cyclopentanes and an aromatic amine.
The clinical success of multitargeted kinase inhibitors has stimulated efforts to identify promiscuous drugs with optimal selectivity profiles. It remains unclear to what extent such drugs can be rationally designed, particularly for combinations of targets that are structurally divergent. Here we report the systematic discovery of molecules that potently inhibit both tyrosine kinases and phosphatidylinositol-3-OH kinases, two protein families that are among the most intensely pursued cancer drug targets. Through iterative chemical synthesis, X-ray crystallography and kinome-level biochemical profiling, we identified compounds that inhibit a spectrum of new target combinations in these two families. Crystal structures revealed that the dual selectivity of these molecules is controlled by a hydrophobic pocket conserved in both enzyme classes and accessible through a rotatable bond in the drug skeleton. We show that one compound, PP121, blocks the proliferation of tumor cells by direct inhibition of oncogenic tyrosine kinases and phosphatidylinositol-3-OH kinases. These molecules demonstrate the feasibility of accessing a chemical space that intersects two families of oncogenes.[1] Here we explored the potential effect of PP121, a novel dual inhibitor of tyrosine and phosphoinositide kinases, against human esophageal cancer cells. We showed that PP121 exerted potent cytotoxic effect in primary (patient-derived) and established (Eca-109, TE-1 and TE-3 lines) esophageal cancer cells, possibly through activating caspase-3-dependnent apoptosis. PP121 was, however, non-cytotoxic to the normal human esophageal epithelial cells (EECs). At the molecular level, we showed that PP121 blocked Akt-mTOR (mammalian target of rapamycin) activation in esophageal cancer cells, which was restored by introducing a constitutively-active Akt (CA-Akt). Yet, CA-Akt only partly inhibited cytotoxicity by PP121 in Eca-109 cells. Importantly, we showed that PP121 inhibited nuclear factor kappa B (NFκB) signaling activation in esophageal cancer cells, which appeared independent of Akt-mTOR blockage. In vivo, oral administration of PP121 remarkably inhibited Eca-109 xenograft growth in nude mice, and significantly improved mice survival. Further, the immunohistochemistry (IHC) and Western blot assays analyzing xenografted tumors showed that PP121 inhibited Akt-mTOR and NFκB activations in vivo. Together, we demonstrate that PP121 potently inhibits esophageal cancer cells in vitro and in vivo, possibly through concurrently inhibiting Akt-mTOR and NFκB signalings.[2] |
| 分子式 |
C17H17N7
|
|---|---|
| 分子量 |
319.36378
|
| 精确质量 |
319.154
|
| 元素分析 |
C, 63.93; H, 5.37; N, 30.70
|
| CAS号 |
1092788-83-4
|
| 相关CAS号 |
1092788-83-4
|
| PubChem CID |
24905142
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.63
|
| 沸点 |
650.9±50.0 °C at 760 mmHg
|
| 闪点 |
347.4±30.1 °C
|
| 蒸汽压 |
0.0±1.9 mmHg at 25°C
|
| 折射率 |
1.881
|
| LogP |
2.41
|
| tPSA |
98.3
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
454
|
| 定义原子立体中心数目 |
0
|
| SMILES |
NC1=C2C(C3=CC4=C(N=C3)NC=C4)=NN(C2=NC=N1)C5CCCC5
|
| InChi Key |
NVRXTLZYXZNATH-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C17H17N7/c18-15-13-14(11-7-10-5-6-19-16(10)20-8-11)23-24(12-3-1-2-4-12)17(13)22-9-21-15/h5-9,12H,1-4H2,(H,19,20)(H2,18,21,22)
|
| 化学名 |
1-cyclopentyl-3-(1H-pyrrolo[2,3-b]pyridin-5-yl)pyrazolo[3,4-d]pyrimidin-4-amine
|
| 别名 |
PP121; PP-121; PP121; 1092788-83-4; 1-cyclopentyl-3-(1H-pyrrolo[2,3-b]pyridin-5-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine; 1-cyclopentyl-3-(1H-pyrrolo[2,3-b]pyridin-5-yl)pyrazolo[3,4-d]pyrimidin-4-amine; CHEBI:50915; 5B9VB06146; PP 121
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~64 mg/mL (~200.4 mM)
Water: <1 mg/mL Ethanol: ~2 mg/mL (~6.3 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2 mg/mL (6.26 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2 mg/mL (6.26 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.0mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2 mg/mL (6.26 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1313 mL | 15.6563 mL | 31.3126 mL | |
| 5 mM | 0.6263 mL | 3.1313 mL | 6.2625 mL | |
| 10 mM | 0.3131 mL | 1.5656 mL | 3.1313 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
 |
|---|
 |