| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
体外活性:盐酸普鲁卡因酰胺是一种抗心律失常药,用于治疗心律失常;诱导快速阻断由蝙蝠毒素 (BTX) 激活的心肌钠通道,并作为长期门控关闭的拮抗剂。
|
||
|---|---|---|---|
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
75 to 95% Trace amounts may be excreted in the urine as free and conjugated p-aminobenzoic acid, 30 to 60 percent as unchanged PA, and 6 to 52 percent as the NAPA derivative. 2 L/kg PROCAINAMIDE IS RAPIDLY & ALMOST COMPLETELY ABSORBED FROM GI TRACT. WHEN... GIVEN ORALLY, ITS PLASMA CONCN BECOMES MAX IN ABOUT 60 MIN; AFTER IM ADMIN PEAK PLASMA CONCN ARE REACHED IN 15-60 MIN. AT ORDINARY PLASMA CONCN, ONLY 15%...IS BOUND TO MACROMOLECULAR CONSTITUENTS OF PLASMA. CONCN OF DRUG IN MOST TISSUES EXCEPT BRAIN IS GREATER THAN THAT IN PLASMA. APPROX 60% OF DRUG IS EXCRETED BY KIDNEY. TWO TO 10%...IS RECOVERED IN URINE AS FREE & CONJUGATED P-AMINOBENZOIC ACID. CONSIDERABLE DIFFERENCES...OBSERVED IN PROCAINAMIDE & ALSO PROCAINAMIDE ETHOBROMIDE DISPOSITION. ETHOBROMIDE IS CLEARED RAPIDLY IN BILE OF RATS & RABBITS, BUT ONLY SLOWLY IN DOGS. 64% OF ORALLY DOSED PROCAINAMIDE IS VOIDED AS UNCHANGED...IN HUMAN URINE, WHEREAS IN RHESUS MONKEY IT IS ALMOST COMPLETELY METABOLIZED. RENAL, CARDIAC, OR HEPATIC IMPARIMENT...RESULTED IN PROLONGED PLASMA HALF-LIVES, & THERE IS EVIDENCE THAT PROCAINAMIDE MAY INHIBIT ITS OWN ELIMINATION AFTER MULTIPLE DOSING. For more Absorption, Distribution and Excretion (Complete) data for PROCAINAMIDE (7 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic DRUG IS RELATIVELY SLOWLY HYDROLYZED BY PLASMA ESTERASES.../PRC: AND BY MICROSOMAL ENZYMES/ PROCAINAMIDE...WAS METABOLIZED TO N-ACETYL DERIV FOLLOWING ORAL ADMIN TO MAN & RHESUS MONKEYS. ... TWO MAJOR METABOLITES WERE DETECTED IN MONKEY URINE, P-ACETAMIDOBENZOIC ACID & DE-ETHYLATED DERIV, P-ACETAMIDO-N-[2-(ETHYLAMINO)-ETHYL]BENZAMIDE. N-ACETYLPROCAINAMIDE IS AN ACTIVE METABOLITE OF PROCAINAMIDE. Procainamide has known human metabolites that include Acecainide. Biological Half-Life ~2.5-4.5 hours PLASMA HALF-LIVES OF PARENT DRUG & ITS TWO MAJOR METABOLITES IN MAN IS BETWEEN 2 & 3 HR. ELIMINATION IS DIRECTLY RELATED TO CREATININE CLEARANCE, & PLASMA HALF-LIFE OF UNCHANGED DRUG IS CONSIDERABLY INCR IN CASES OF RENAL IMPAIRMENT. ...BIOLOGICAL HALF-LIFE IS 3-4 HR... |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION: Procainamide is an antiarrhythmic medication. Procainamide hydrochloride is a white to tan colored hygroscopic odorless crystalline powder. Soluble in water, alcohol, chloroform and practically insoluble in ether and benzene HUMAN EXPOSURE: Main risks and target organs: The heart is the main target organ. Procainamide is an antiarrhythmic agent used to suppress ventricular tachydysrhythmias. It increases the effective refractory period of the atria, and (to a lesser extent) that of the bundle of the His-Purkinje system and the ventricles. Toxic effects result from delay in conduction and depression of myocardial contractility, leading to cardiac dysrhythmia and cardiogenic shock. Its oral use is limited immunological adverse effects such as systemic lupus erythematosus in patients on chronic oral therapy. Summary of clinical effects: Cardiovascular System: Sinus or atrial tachycardias, atrioventricular and intraventricular block hypotension, cardiogenic shock, torsades de pointes and ventricular fibrillation. Central Nervous System: Lethargy, coma, respiratory arrest may result Gastrointestinal Tract: Nausea, vomiting, diarrhea and abdominal pain have been reported. Others: Anticholinergic effects, hypokalemia, metabolic acidosis and pulmonary edema. Indications: Suppression of ventricular arrhythmias. Treatment of automatic and reentrant supraventricular tachycardia. Supraventricular arrhythmias: Like quinidine, procainamide is only moderately effective in converting atrial flutter or chronic atrial fibrillation to sinus rhythm. The drug can be used to prevent recurrences of atrial flutter or atrial fibrillation after cardioversion. Procainamide is indicated in the treatment of ventricular premature contractions, and in preventing recurrence of ventricular tachycardia after conversion to sinus rhythm by intravenous drugs or by electrical cardioversion or by other antiarrhythmic therapy; also in preventing recurrence of paroxysmal supraventricular tachycardia, atrial fibrillation or flutter following conversion to sinus rhythm by initial vagotonic maneuvers, digitalis preparations, other pharmaceutical antiarrhythmic agents, or electrical cardioversion. The drug is useful in patients with severe ventricular arrhythmias who do not respond to lidocaine. Procainamide is useful for acute terminations of arrhythmias associated with the Wolff-Parkinson-White Syndrome. Procainamide is used in the treatment of cardiac arrhythmias occurring in patients during general anesthesia. The drug has been used in conjunction with hexamethonium bromide to produce controlled hypotension and, consequently, ischemia of sufficient degree for relatively bloodless field surgery. The injection of procainamide into painful soft tissues in fibrosis and radiculitis and into the periarticular tissues in degenerative arthritis provided relief for considerable periods. Contraindications: Complete heart block: because of its effects in suppressing nodal or ventricular pacemakers. Torsades de Pointes: administration of procainamide in such case may aggravate this special type of ventricular extrasystole or tachycardia instead of suppressing it. Idiosyncratic hypersensitivity: in patients sensitive to procaine or other ester-type local anesthetics, cross sensitivity to procainamide is unlikely. However, a previous allergic reaction to procainamide is a contraindication. Lupus erythematosus: aggravation of symptoms is highly likely. Precautions: Preferably, procainamide should not be used in patients with bronchial asthma or myasthenia gravis. Accumulation of the drug may occur in patients with heart, renal or liver failure. Procainamide may enhance the effects of antihypertensive agents, propranolol, and some skeletal muscle relaxants. Grave hypotension may follow intravenous administration of procainamide; it should be injected slowly under monitoring of blood pressure and ECG. Although procainamide has been used effectively in the treatment of ventricular dysrhythmias caused by digitalis intoxication, its effects are unpredictable and fatalities have occurred. Procainamide should not be administered to nursing mothers. Routes of entry: Oral: Oral route is a common route of entry in cases of poisoning. Parenteral: Toxicity reactions can occur after intravenous injections. Absorption by route of exposure: Oral: Procainamide is almost completely and rapidly absorbed from the gastrointestinal tract. Peak levels are reached within 1 hr after ingestion of capsules, but somewhat later after administration of tablets. The bioavailability is approximately 85%. An overdose may significantly delay intestinal procainamide absorption and prolong poisoning symptoms. With the sustained-release formulations, bioavailability is decreased and the absorption is delayed. The duration of action exceeds 8 hours. Intramuscular: Plasma concentrations showed very large variations. Procainamide appears in the plasma within two minutes and peak concentrations are reached within 25 minutes. Intravenous: Procainamide acts almost immediately, the plasma level declines 10 to 15% hourly. Distribution by route of exposure: About 20% of the procainamide in plasma is bound to proteins. Procainamide is rapidly distributed into most body tissues except the brain. In patients with cardiac failure or shock the volume of distribution may decrease. Procainamide crosses the placental barrier and has been reported to accumulate in the fetus. Biological half-life by route of exposure: Peak plasma levels: Oral: one to 2 hours after ingestion. Intramuscular: eighty minutes after administration. Intravenous: Within several minutes. The plasma half life after therapeutic doses is 3 to 4 hours. However, in one patient the overdose plasma half life was 8.8 hours. Congestive heart failure increases the plasma procainamide half life to 5 to 8 hours. The half-life is reduced in children and is prolonged in patients with renal insufficiency. Its major active metabolite, N-acetylprocainamide (NAPA), has a longer half-life than procainamide, from 6 hours up to 36 hours in overdoses. Metabolism: The major metabolic pathway of procainamide is hepatic N-acetylation. The rate of acetylation is determined genetically and shows a bimodal distribution into slow and fast acetylators. The major active metabolite, NAPA, has antiarrhythmic properties. Other urinary metabolites include desethyl-NAPA and desethyl-procainamide, which account for 8 to 15% of a dose of procainamide. The exact relationship between antiarrhythmic activity and plasma levels of NAPA has not been established. Up to 15% of the intravenous procainamide therapeutic dose is metabolized to NAPA, and 81% of the NAPA dose is excreted unchanged in urine. In fast acetylators or in renal insufficiency, 40% or more of a dose of procainamide may be excreted as NAPA, and its concentrations in plasma may equal or exceed those of the parent drug. Procainamide hydrochloride is only slightly hydrolyzed by plasma enzymes (to p-amino benzoic acid and diethylaminoethylamine). Elimination by route of exposure: Procainamide is excreted in the urine with about 50% as unchanged procainamide, and up to about 30% as NAPA (less in slow acetylators). Since the elimination of both the parent drug and metabolites is almost entirely by renal excretion, they can accumulate to dangerous levels when renal failure or congestive heart failure is present. After an overdose, hepatic biotransformation probably is a more important elimination pathway than renal excretion. Following an overdose, the elimination half-life (in the presence of a serum creatinine of 5.8 mg/dL) of NAPA increased from 6 to 35.9 hours while the procainamide elimination increased from 3 to 10.5 hours. Mode of action: Toxicodynamics: Toxic effects result from quinidine-like effect with delay of conduction and depression of myocardial contractility. Contractility of the undamaged heart is usually not affected by therapeutic concentrations, although slight reduction of cardiac output may occur, and may be significant in the presence of myocardial damage. High toxic concentrations may prolong atrioventricular conduction time or induce atrioventricular block or even cause abnormal automaticity and spontaneous firing, by unknown mechanisms. The toxic mechanism of the drug is dose dependent and is related to depression of contractility, decreased vascular resistance secondary to direct vasodilatation and some alpha adrenergic blocking. Besides the cardiovascular effects, procainamide produces CNS depression and has anticholinergic effects. Pharmacodynamics: Procainamide is an antiarrhythmic agent with electrophysiological properties similar to that of quinidine. Procainamide increases the effective refractory period of the atria, of the bundle of His-Purkinje system and of the ventricles. It reduces impulse conduction velocity in atria, His-Purkinje fibers, and ventricular muscle. But it has also variable effects on the atrioventricular node, a direct slowing action and a weaker vagolytic effect which may speed atrio-conduction slightly. Myocardial excitability is reduced in the atria, Purkinje fibers, papillary muscles, and ventricles by an increase in the threshold for excitation. NAPA is less potent than procainamide, and some of its cardiac actions are qualitatively different. Procainamide does not produce alpha-adrenergic blockade, but, in the dog, it can block autonomic ganglia weakly and cause a measurable impairment of cardiovascular reflexes. Human data: Adults: A single oral dose may produce symptoms of toxicity. Ingestion of 3 gm may be dangerous, especially if patient is slow acetylator or has renal impairment or underlying heart disease. Death was reported from intravenous administration. Interactions: If other antiarrhythmic drugs are being used, additive effects on the heart may occur with procainamide administration, and dosage reduction may be necessary. Anticholinergic drugs administered concurrently with procainamide may produce additive antivagal effects on A-V nodal conduction. Patients taking procainamide who require neuromuscular blocking agents such as succinylcholine may require less than usual doses of the latter, due to procainamide effect on reducing acetylcholine release. The neuromuscular blocking activity of an antibiotic having such action may be accentuated by procainamide. The hypotensive action of antihypertensive agents, including thiazide diuretics, may be potentiated by procainamide. Cimetidine therapy given to older male patients taking procainamide may increase steady-state concentrations of procainamide. Main adverse effects: The side-effects most frequently reported after high dosage of procainamide include anorexia, diarrhea, nausea, and vomiting. Intravenous administration may cause hypotension, ventricular fibrillation or asystole if the injection is too rapid. Following chronic administration, systemic lupus erythematosus-like syndrome may develop. Other side effects which have been reported include mental depression, dizziness, psychosis with hallucinations, joint and muscle pain, muscular weakness, a bitter taste, flushing, skin rashes, pruritus, angioneurotic edema and hypersensitivity leading to chills, fever and urticaria. Leucopenia and agranulocytosis have followed repeated use of procainamide. Neutropenia, thrombocytopenia, or hemolytic anemia may rarely be encountered. High concentrations of procainamide in plasma can produce ventricular premature depolarization, ventricular tachycardia, or ventricular fibrillation. Hepatomegaly with increased serum aminotransferase level has been reported after a single oral dose. Mild hypovolemia, hypokalemia, metabolic acidosis may occur. Increased QT interval and prolonged QRS together with hypotension are sensitive indexes of serious poisoning. Parenteral administration of procainamide should be monitored electrocardiographically to give evidence of impending heart block. Acute poisoning: Ingestion: Serious toxic effects include conduction disturbances (QRS, QT prolongations), ventricular arrhythmias and cardiogenic shock. Increased ventricular extrasystoles, ventricular tachycardia (especially of the "torsades de pointes" type) or fibrillation may occur. The threshold of cardiac pacing is increased and the heart may even be nonresponsive. Lethargy, confusion and coma may occur. Other toxic manifestations are pulmonary edema, respiratory depression, urticaria, pruritus, nausea, vomiting, diarrhea and abdominal pain. Psychosis with hallucinations have been reported occasionally. Parenteral exposure: Intravenous administration may cause hypotension, ventricular fibrillation or asystole if the injection is too rapid. Chronic poisoning: Ingestion: A lupus erythematosus like syndrome of arthralgia, pleural or abdominal pain, and sometimes arthritis, pleural effusion, pericarditis, fever, chills, myalgia, and possibly related hematologic or skin lesions is fairly common after prolonged procainamide administration. Neutropenia, thrombocytopenia, or hemolytic anemia may rarely be encountered. Agranulocytosis has occurred after repeated use of procainamide. Course, prognosis, cause of death: Presence of PVCs and runs of ventricular tachycardia that are almost always successfully treated. Prognosis is usually good if there is not progress to ventricular fibrillation or asystole. Death is due to ventricular fibrillation or asystole. Long-term effects are agranulocytosis from hypersensitivity reaction, which is associated with 90% recovery rate. Systematic description of clinical effects: Cardiovascular: Acute: Sinus or atrial tachycardia due to the vagolytic effects. Conduction disturbances such as atrioventricular block, intraventricular block. Ventricular arrhythmias, including torsades de pointes, ventricular tachycardia and fibrillation. Hypotension and cardiogenic shock. ECG may show widening QRS, atrioventricular block, prolongation of QT interval and ventricular arrhythmia. Chronic: Chronic exposure may also produce arrhythmias. Cardiac tamponade due to pericarditis has been reported in a case of procainamide induced systemic lupus syndrome. Respiratory: Acute: Respiratory arrest and pulmonary edema. Neurological: Central nervous system (CNS): Acute: Dizziness or giddiness, weakness, mental depression, and psychosis with hallucinations have been reported occasionally. Lethargy may progress to coma. Skeletal and smooth muscle: Chronic: Skeletal muscular weakness and diaphragmatic paralysis has been reported in a case. Gastrointestinal: Acute: Anorexia, nausea, vomiting, abdominal pain, bitter taste, or diarrhea may occur in 3 to 4% of patients taking oral procainamide. Chronic: Nausea, vomiting may be seen. Hepatic: Acute: Hepatomegaly with increased serum aminotransferase level has been reported after a single oral dose. Dermatological: Chronic: Angioneurotic edema, urticaria, pruritus, flushing, and maculopapular rashes. Eye, ear, nose, throat: local effects: Acute: Blurred vision has been reported. Hematological: Chronic: Neutropenia, thrombocytopenia, or hemolytic anemia and agranulocytosis may rarely be encountered. Immunological: Chronic: Systemic lupus erythematosus like syndrome has been reported. Metabolic: Acid-base disturbances: Acute: Metabolic acidosis has been reported. Fluid and electrolyte disturbances: Acute: Hypokalemia may occur. Angioneurotic edema and maculopapular rashes have been reported. Special risks: Pregnancy: It is not known whether procainamide cause fetal harm when administered to a pregnant woman. Procainamide should be given to a pregnant woman only if clearly needed. Breast feeding: Both procainamide and NAPA are excreted in human milk. Therefore, procainamide should be given to a nursing mother only if clearly needed. Pediatric use: Safety and effectiveness in children have not been established. Plasma levels of NAPA may rise disproportionately in patients with renal impairment, because it is more dependent than procainamide on renal excretion for elimination. Elimination: Renal elimination of procainamide appears not to be affected by urinary pH or by urinary flow rate. However, because procainamide and NAPA are substantially eliminated by the kidney, it is important to maintain adequate renal functions. Hepatotoxicity In clinical trials, procainamide was associated with a low rate of serum aminotransferase and alkaline phosphatase elevations. Despite wide scale use, procainamide has only rarely been linked to cases of clinically apparent liver injury. In reported cases, fever and mild symptoms arose within 1 to 3 weeks of starting (or within 1 day of restarting) procainamide, associated with a cholestatic pattern of serum enzyme elevations with mild or no jaundice (Case 1). Immunoallergic features were usually present (fever, rash, leukocytosis). In reported cases, fever resolved immediately and evidence of liver injury within a few days to weeks of stopping procainamide. Liver biopsy may how granulomas in addition to mild nonspecific changes. Interestingly, the hepatotoxicity of procainamide closely resembles that of quinidine, but there is no apparent cross sensitivity to the hepatic injury. In addition, up to 20% of patients on long term procainamide therapy develop autoantibodies, including ANA and LE prep positivity and a proportion develop a “lupus-like” syndrome. These autoimmune conditions, however, typically occur without an accompanying hepatitis, serum enzyme elevations or jaundice. Likelihood score: C (probable rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Maternal doses of procainamide 2 grams daily produced low levels of the drug and its active metabolite in the milk of one mother. Although it would not be expected to cause adverse effects in older breastfed infants, the relative lack of data concerning breastfeeding during maternal procainamide therapy would argue for careful monitoring if this drug is used while breastfeeding a neonate possibly Measurement of infant serum levels could help to rule out toxicity if there is a concern. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding 15 to 20% Interactions STUDIES HAVE DEMONSTRATED THAT PROCAINAMIDE IN CATS...MAY PROLONG & INTENSIFY NEUROMUSCULAR BLOCKADE PRODUCED BY SUCCINYLCHOLINE. |
||
| 参考文献 |
JPharmacol Rep.2016 Jun;68(3):654-61.
|
||
| 其他信息 |
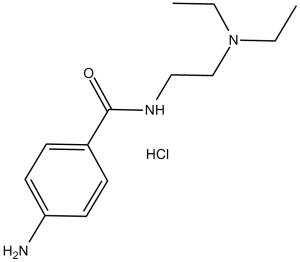
Procainamide is a benzamide that is 4-aminobenzamide substituted on the amide N by a 2-(diethylamino)ethyl group. It is a pharmaceutical antiarrhythmic agent used for the medical treatment of cardiac arrhythmias. It has a role as a sodium channel blocker, an anti-arrhythmia drug and a platelet aggregation inhibitor.
A derivative of procaine with less CNS action. Procainamide is an Antiarrhythmic. Procainamide is an oral antiarrhythmic agent that has been in use for more than 60 years. Long term procainamide therapy is known to induce hypersensitivity reactions, autoantibody formation and a lupus-like syndrome but is a rare cause of clinically apparent acute liver injury. A class Ia antiarrhythmic drug that is structurally-related to PROCAINE. See also: Procainamide Hydrochloride (has salt form). Drug Indication For the treatment of life-threatening ventricular arrhythmias. Mechanism of Action Procainamide is sodium channel blocker. It stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses thereby effecting local anesthetic action. IV ADMIN...CAUSES FALL IN BLOOD PRESSURE; PERIPHERAL VASODILATATION PROBABLY CONTRIBUTES TO HYPOTENSIVE RESPONSE, BUT SYSTOLIC PRESSURE MAY BE REDUCED MORE THAN DIASTOLIC. ... CNS ACTIONS OF PROCAINAMIDE ARE NOT PROMINENT. Therapeutic Uses Anti-Arrhythmia Agents; Platelet Aggregation Inhibitors PROCAINAMIDE IS USEFUL IN SUPPRESSING ARRHYTHMIAS OF VENTRICULAR ORIGIN, INCL VENTRICULAR EXTRASYSTOLES, PAROXYSMAL VENTRICULAR TACHYCARDIA, & VENTRICULAR FIBRILLATION. /HYDROCHLORIDE SALT/ DRUG IS...EFFECTIVE AGAINST PAROXYSMAL ATRIAL TACHYCARDIA, ATRIAL FLUTTER, & ATRIAL TACHYCARDIA OR ATRIAL ECTOPIC SYSTOLES. IN CASES OF PAROXYSMAL ATRIAL TACHYCARDIA, OTHER MEASURES & AGENTS OF CHOICE SHOULD BE EMPLOYED BEFORE PROCAINAMIDE IS USED. /HYDROCHLORIDE SALT/ PROCAINAMIDE HAS BEEN USED IN TREATMENT OF MYOTONIA, WHERE ITS EFFECTS RESEMBLE THOSE OF QUININE. For more Therapeutic Uses (Complete) data for PROCAINAMIDE (8 total), please visit the HSDB record page. Drug Warnings ...USED WITH CAUTION & MEDICATION MUST BE STOPPED IF QRS COMPLEX IS EXCESSIVELY WIDENED. PROCAINAMIDE IS USUALLY WELL TOLERATED. HOWEVER, IT HAS OCCASIONALLY CAUSED SERIOUS SIDE EFFECTS, & DEATHS HAVE RESULTED. ...PROCAINAMIDE CAN CAUSE UNTOWARD RESPONSES BY ITS ACTIONS ON ABNORMAL MYOCARDIUM OR AS RESULT OF CORRECTION OF ARRHYTHMIAS FOR WHICH DRUG IS ADMIN. ... PROCAINAMIDE...SHOULD NOT BE ADMIN WHEN COMPLETE A-V BLOCK IS PRESENT & SHOULD BE USED ONLY CAUTIOUSLY IN PRESENCE OF PARTIAL BLOCK BECAUSE OF DANGER OF ASYSTOLE. CROSS SENSITIVITY TO PROCAINE & RELATED DRUGS SHOULD BE ANTICIPATED. ... /IT/...MUST BE GIVEN CAUTIOUSLY IF PT IS DIGITALIZED. /HCL SALT/ FATAL AGRANULOCYTOSIS...REPORTED, & FREQUENT BLOOD EXAM DURING CHRONIC... THERAPY ARE ESSENTIAL. SYNDROME SIMILAR TO SYSTEMIC LUPUS ERYTHEMATOSUS IS COMMON REACTION TO CHRONIC ADMIN, & MAY NECESSITATE TERMINATION OF THERAPY... For more Drug Warnings (Complete) data for PROCAINAMIDE (9 total), please visit the HSDB record page. Pharmacodynamics Procainamide is an agent indicated for production of local or regional anesthesia and in the treatment of ventricular tachycardia occurring during cardiac manipulation, such as surgery or catheterization, or which may occur during acute myocardial infarction, digitalis toxicity, or other cardiac diseases. The mode of action of the antiarrhythmic effect of Procainamide appears to be similar to that of procaine and quinidine. Ventricular excitability is depressed and the stimulation threshold of the ventricle is increased during diastole. The sinoatrial node is, however, unaffected. |
| 分子式 |
C13H22CLN3O
|
|
|---|---|---|
| 分子量 |
271.79
|
|
| 精确质量 |
271.145
|
|
| CAS号 |
614-39-1
|
|
| 相关CAS号 |
Procainamide;51-06-9
|
|
| PubChem CID |
4913
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 沸点 |
421.8ºCat 760 mmHg
|
|
| 熔点 |
165-168 °C
|
|
| 闪点 |
208.9ºC
|
|
| LogP |
3.114
|
|
| tPSA |
58.36
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
6
|
|
| 重原子数目 |
17
|
|
| 分子复杂度/Complexity |
221
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
Cl[H].O=C(C1C([H])=C([H])C(=C([H])C=1[H])N([H])[H])N([H])C([H])([H])C([H])([H])N(C([H])([H])C([H])([H])[H])C([H])([H])C([H])([H])[H]
|
|
| InChi Key |
ABTXGJFUQRCPNH-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C13H21N3O.ClH/c1-3-16(4-2)10-9-15-13(17)11-5-7-12(14)8-6-11;/h5-8H,3-4,9-10,14H2,1-2H3,(H,15,17);1H
|
|
| 化学名 |
4-amino-N-[2-(diethylamino)ethyl]benzamide;hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 3.25 mg/mL (11.96 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 32.5 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 3.25 mg/mL (11.96 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 32.5 mg/mL 澄清 DMSO 储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 3.25 mg/mL (11.96 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 120 mg/mL (441.52 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.6793 mL | 18.3966 mL | 36.7931 mL | |
| 5 mM | 0.7359 mL | 3.6793 mL | 7.3586 mL | |
| 10 mM | 0.3679 mL | 1.8397 mL | 3.6793 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02103088 | Completed | Behavioral: Sexual and urological intervention |
Prostate Cancer | Danish Cancer Society | May 2014 | Not Applicable |
| NCT02739932 | Completed | Psychotic Disorders Depressive Disorder, Major |
University of Calgary | March 2015 | ||
| NCT02575534 | Withdrawn | Drug: Procainamide | Arrhythmias | Evan Adelstein, MD | October 2015 | Not Applicable |
| NCT02114528 | Terminated | Drug: Antiarrhythmic Drug Therapy Procedure: Catheter ablation |
Ventricular Tachycardia Ventricular Arrhythmia |
Ottawa Heart Institute Research Corporation |
October 2014 | Phase 4 |