| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| Other Sizes |
| 靶点 |
hGAT-1 (IC50 = 0.26 μM); rGAT-1 (IC50 = 1.2 μM); rGAT-2 (IC50 = 297 μM); hGAT-3 (IC50 = 333 μM); rGAT-3 (IC50 = 1140 μM); hBGT-3 (IC50 = 300 μM)
|
|---|---|
| 体外研究 (In Vitro) |
CI-966 盐酸盐的作用机制是特异性阻断神经元和神经胶质细胞中的 GABA 再摄取[4]。
γ-氨基丁酸(GABA)是哺乳动物大脑中主要的抑制性神经递质。GABA的突触作用被突触前终末和周围神经胶质细胞的快速摄取终止。分子克隆揭示了四种不同的GABA转运蛋白的存在,称为GAT-1、GAT-2、GAT-3和BGT-1。运输的药理学抑制提供了一种增加GABA能量传递的机制,这可能有助于治疗各种神经精神疾病。最近,已经设计并合成了一些亲脂性GABA转运抑制剂,它们能够穿过血脑屏障,并显示出抗惊厥活性。我们现在已经测定了其中四种化合物的效力,SK&F 89976-A(N-(4,4-二苯基-3-丁烯基)-3-哌啶甲酸)、噻加宾((R)-1-[4,4-双(3-甲基-2-噻吩基)-3-丁烯基]-3-哌啶甲酸),CI-966([1-[2-[双4-(三氟甲基)苯基]甲氧基]乙基]-1,2,5,6-四氢-3-吡啶甲酸)和NNC-711(1-(2-(((二苯基亚甲基)氨基)氧基)乙基)-1,2,4,6-四氢-3-吡啶甲酸盐酸盐),并发现它们对GAT-1具有高度选择性。这些数据表明,这些化合物的抗惊厥活性是通过抑制GAT-1的摄取来介导的[1]。 分子生物学揭示了大脑中存在四种高亲和力GABA转运蛋白,即GAT-1、GAT-2、GAT-3和BGT-1,后者运输GABA和渗透调节剂甜菜碱。我们已经证明,已知的GABA摄取抑制剂如SK&F 89976-A、CI-966和Tiagabine对GAT-1表现出高亲和力和选择性[3]。 |
| 体内研究 (In Vivo) |
当给予经过 PTZ 训练的大鼠时,CI-966盐酸盐会引起中等水平的戊四氮 (PTZ) 杠杆反应[4]。 CI-966 盐酸盐原位增强 CA1 锥体层中的 γ-氨基丁酸活性。在乌拉坦麻醉下,通过腹膜内注射向 Sprague-Dawley 大鼠全身注射 CI-966 盐酸盐(5 mg/kg)。注射后 20 至 30 分钟,CA1 区域微离子导入 GABA 对海马群体峰值的抑制作用变化很大,但往往相当大[5]。在许多动物模型中,CI-966 盐酸盐表现出抗惊厥特性。当喂食 1.39 mg/kg 时,狗口服吸收 CI-966 盐酸盐,达峰时间为 0.7 小时。大鼠口服 5 mg/kg 的平均 tmax 为 4.0 小时。静脉注射相同剂量后,大鼠和狗的平均消除t1/2分别为4.5小时和1.2小时。在这两个物种中,CI-966 盐酸盐的口服生物利用度均为 100%[6]。
在氨基甲酸乙酯麻醉下,通过腹腔注射(5mg/kg)对Sprague-Dawley大鼠全身施用一种新的强效、可渗透血脑屏障的γ-氨基丁酸(GABA)摄取阻断剂1-[2-[双[4-(三氟甲基)-苯基]甲氧基]乙基]-1,2,5,6-四氢-3-吡啶甲酸(CI-966)。注射后20至30分钟,通过微离子电渗疗法在CA1区施加GABA,对海马群体尖峰的抑制作用发生了高度变化,但总体上显著增强。与尼泊苷酸(通过离子电渗疗法局部应用)的效果一样,当GABA施加在金字塔层或其附近时,CI-966的增强作用最为明显,因为GABA的作用通常最弱,并且表现出最明显的消退。GABA效力的这种变化最简单的解释是GABA摄取的减少。[5] CI-966在各种动物模型中表现出抗惊厥特性。该药物通过抑制γ-氨基丁酸(GABA)的突触摄取起作用。狗口服1.39mg/kg的CI-966吸收迅速,tmax为0.7小时。大鼠口服5mg/kg的平均tmax为4.0小时。静脉注射相同剂量后,狗和大鼠的消除t1/2平均为1.2和4.5小时。两种物种的CI-966的绝对口服生物利用度均为100%。狗口服[14C]CI-966 HCl后,粪便和尿液排泄分别占14C剂量的89%和2.3%。在胆管插管的大鼠中,胆汁排泄是放射性的主要消除途径(75%)。尿液和粪便排泄分别占4.1%和12%。根据己巴比妥睡眠时间的测定,CI-966不会诱导或抑制小鼠肝脏混合功能氧化酶[6]。 |
| 细胞实验 |
细胞系。[3]
在本研究中,我们使用了大鼠GAT-2(rGAT-2)6和GAT-1的人类同源物(我们重新克隆的hGAT-1)、20个GAT-3(hGAT-3)、9和BGT-1(hBGT-1)。如前所述,使用磷酸钙法和G-418中的选择在LM(tk“)细胞中产生了这些克隆的稳定细胞系。细胞在Dulbecco改良的Eagles培养基中在标准条件下(37°C,5%COa)生长。 运输分析。[3] GABA转运如前所述进行测量,6但有以下修改。用HEPES缓冲盐水(HBS,单位为mm:NaCl,150;HEPES,20;CaCla,1;葡萄糖,10;KC1,5;MgCla,l;pH7.4)洗涤在24孔板(孔直径18 mm)中生长的细胞三次,并在37°C的玻片加热器上平衡。10分钟后,取出培养基,加入HBSure中未标记的药物(450/μL/孔)。通过在每孔中加入50 pL的[3H]GABAin-HBS浓缩溶液(终浓度=50 nM)来启动转运。非特异性摄取在具有1个未标记GABA的平行孔中定义,并从总摄取量(无竞争对手)中减去产生特异性摄取量;所有数据均代表特定摄取量。将板在37°C下孵育10分钟,然后使用24位洗板机用冰冷的HBS快速洗涤三次。用0.05%脱氧胆酸钠/0.1 N NaOH(0.25 mL/孔)溶解细胞,用1 N HCl中和等分试样,通过闪烁计数测定放射性。根据制造商的指示,使用BIO-RAD蛋白质测定试剂盒对溶解细胞的等分试样中的蛋白质进行定量。将亲脂性抑制剂溶解在DMSO中。转运分析中DMSO的最终浓度<2%,对照实验表明,该浓度对转运没有显著影响。 |
| 动物实验 |
Animal/Disease Models: Eight male SD (Sprague-Dawley) rats[4]
Doses: 0.3-30 mg/kg Route of Administration: Injection IP in a volume of 1 mL/kg Experimental Results: Dose dependent decreases in rates of responding occurred following CI-966 administration. |
| 药代性质 (ADME/PK) |
Oral absorption of CI-966 in dogs given 1.39 mg/kg is rapid with a tmax of 0.7 hr. In rats given 5 mg/kg oral, a mean tmax of 4.0 hr was observed. Following iv administration of the same respective doses, elimination t1/2 in dogs and rats averaged 1.2 and 4.5 hr. Absolute oral bioavailability of CI-966 was 100% in both species. Following oral dosing of [14C]CI-966 HCl to dogs, fecal, and urinary excretion accounted for 89% and 2.3% of the 14C dose, respectively. In bile-duct cannulated rats, biliary excretion is the major elimination pathway of radioactivity (75%). Urinary and fecal excretion accounted for 4.1 and 12%, respectively. CI-966 does not induce or inhibit mouse hepatic mixed function oxidases, as determined by hexobarbital sleeping time.[6]
|
| 毒性/毒理 (Toxicokinetics/TK) |
198692 rat LD50 oral 894 mg/kg SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE; BEHAVIORAL: COMA; GASTROINTESTINAL: HYPERMOTILITY, DIARRHEA Drug Development Research., 28(65), 1993
198692 mouse LD50 oral 703 mg/kg BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD; GASTROINTESTINAL: HYPERMOTILITY, DIARRHEA; KIDNEY, URETER, AND BLADDER: OTHER CHANGES Drug Development Research., 28(65), 1993 |
| 参考文献 |
|
| 其他信息 |
It has been shown that enhancing the function of the major inhibitory neurotransmitter GABA decreases glutamatergic activity in the brain. Since increased glutamatergic activity is the major primary event that results in cell death following an acute hypoxic-ischaemic stroke, GABAmimetic drugs might therefore be expected to be neuroprotective. This review examines the evidence that GABAergic function is acutely depressed following an ischaemic insult, and also reviews the data that suggest that increasing cerebral GABA concentration has a neuroprotective effect, as does the administration of some (but not all) GABAmimetic agents. The GABA uptake inhibitor CI-966, the GABA(A) agonist muscimol and the GABA(A)mimetic clomethiazole have all been shown to be neuroprotective in animal models of stroke when given after the ischaemic insult. In contrast, benzodiazepines and particularly barbiturates, although potent GABA(A) potentiators, have shown little promise as neuroprotectants. The diversity of GABA(A) receptor subtypes and the in vivo efficacy of certain GABA(A) receptor ligands in animal models of stroke suggests that GABAmimetic drugs are an undervalued approach to stroke therapy.[2]
gamma-Aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the mammalian central nervous system. Molecular biology has revealed the presence of four high-affinity GABA transporters in the brain, GAT-1, GAT-2, GAT-3, and BGT-1, the latter transporting both GABA and the osmolyte Betaine. We have shown that known GABA uptake inhibitors such as SK&F 89976-A, CI-966, and Tiagabine exhibit high affinity and selectivity for GAT-1. In the present paper we describe the design and synthesis of a novel series of triarylnipecotic acid derivatives for evaluation as GABA uptake inhibitors. The design lead for this series of compounds was the nonselective GABA uptake inhibitor EGYT-3886, [(-)-2-phenyl-2-[(dimethylamino)ethoxy]-(1R)- 1,7,7-trimethylbicyclo[2.2.1]heptane]. From this series of compounds (S)-1-[2-[tris(4-methoxyphenyl)methoxy]ethyl]-3-piperidinecarboxylic+ ++ (S)-1-[2-[tris(4-methoxyphenyl)methoxy]ethyl]-3-piperidinecarboxylic+ ++ acid, 4(S) was identified as a novel ligand with selectivity for GAT-3. 4(S) displayed an IC50 of 5 microM at GAT-3, 21 microM at GAT-2, > 200 microM at GAT-1, and 140 microM at BGT-1. This compound will be an important tool for evaluating the role of GAT-3 in neural function.[3] The discriminative stimulus effects of indirect-acting GABAergic drugs were compared to those of pentobarbital (PB) and midazolam in rats trained to discriminate 5 mg/kg PB from saline under a two-lever fixed-ratio 32 schedule of food reinforcement. PB and midazolam produced dose-dependent substitution for the training dose of PB with response rate reduction only at doses above those producing full substitution. Valproic acid, an antiepileptic drug and GABA transaminase inhibitor, substituted for PB but only at a dose that produced response rate suppression. Vigabatrin, an irreversible GABA transaminase inhibitor, failed to substitute for PB, but did produce a dose-dependent decrease in response rates. The GABA uptake inhibitors, 1-[2-[bis[4-(trifluoromethyl)phenyl]-methoxy]ethyl]-1,2,5,6- tetrahydro-3-pyridinecarboxylic acid (CI-966) and (R(-)-N-[4,4-bis(3-methylthien-2-yl)but-3-enyl] nipecotic acid HCl (tiagabine), produced no greater than 40% PB-lever responding. Aminooxyacetic acid (AOAA), which is described as a nonselective presynaptic GABA agonist, yielded a maximum of 43% PB-lever responding. These results indicate that the discriminative stimulus effects of the indirect GABAA agonists, PB and midazolam, although similar to one another, differ from those of presynaptic GABAergic drugs. Differences in the discriminative stimulus properties of GABA transaminase inhibitors and uptake inhibitors also exist, indicating that not all presynaptic GABA agonists have similar behavioral profiles. These results contribute to a further understanding of the similarities and differences in the behavioral effects of drugs that enhance GABAergic neurotransmission.[4] |
| 分子式 |
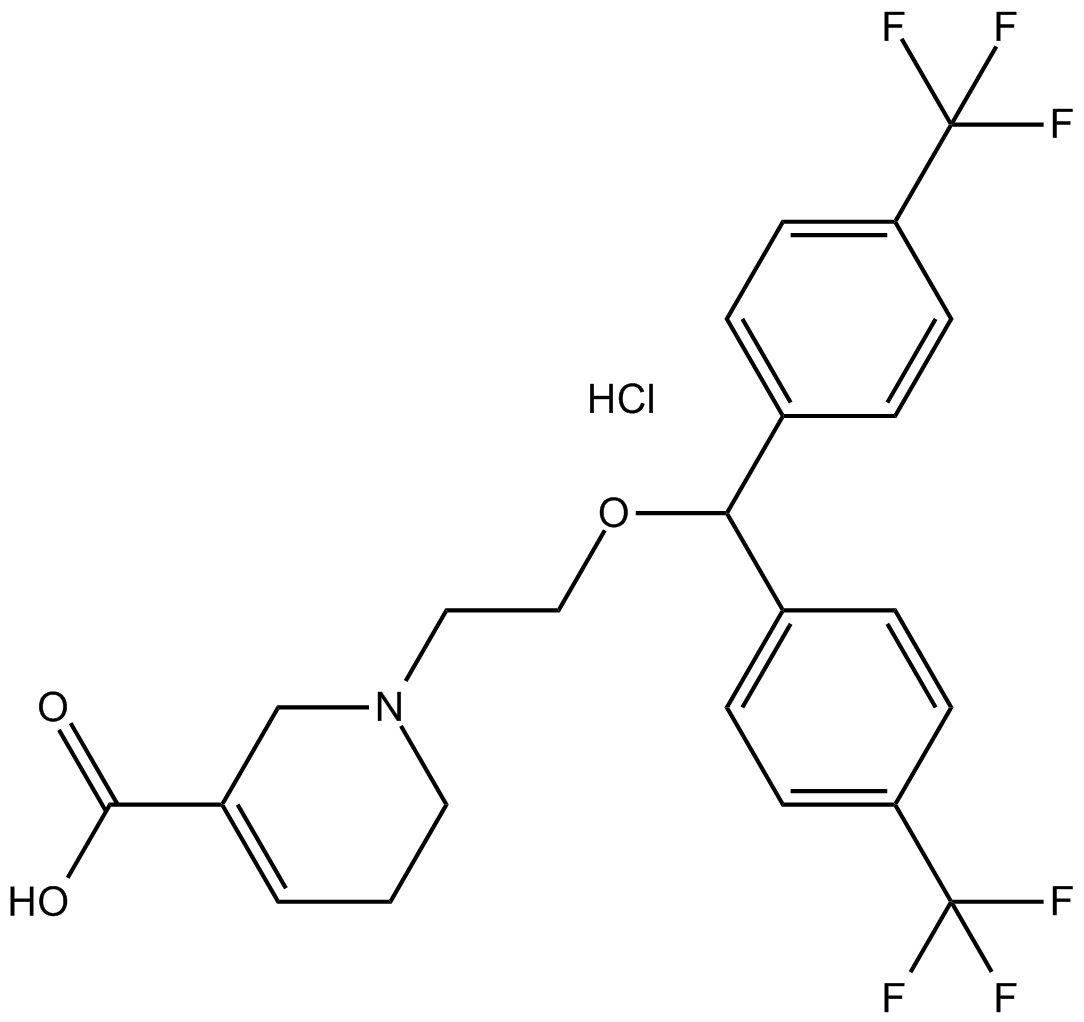
C23H22CLF6NO3
|
|---|---|
| 分子量 |
509.87
|
| 精确质量 |
509.119
|
| 元素分析 |
C, 54.18; H, 4.35; Cl, 6.95; F, 22.36; N, 2.75; O, 9.41
|
| CAS号 |
110283-66-4
|
| 相关CAS号 |
CI-966;110283-79-9
|
| PubChem CID |
198692
|
| 外观&性状 |
White to off-white solid powder
|
| 沸点 |
514.1ºC at 760mmHg
|
| 闪点 |
264.7ºC
|
| 蒸汽压 |
2.16E-11mmHg at 25°C
|
| LogP |
6.286
|
| tPSA |
49.77
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
10
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
34
|
| 分子复杂度/Complexity |
643
|
| 定义原子立体中心数目 |
0
|
| SMILES |
C1CN(CC(=C1)C(=O)O)CCOC(C2=CC=C(C=C2)C(F)(F)F)C3=CC=C(C=C3)C(F)(F)F.Cl
|
| InChi Key |
NUQWSOWKRTZJTO-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C23H21F6NO3.ClH/c24-22(25,26)18-7-3-15(4-8-18)20(16-5-9-19(10-6-16)23(27,28)29)33-13-12-30-11-1-2-17(14-30)21(31)32;/h2-10,20H,1,11-14H2,(H,31,32);1H
|
| 化学名 |
1-[2-[bis[4-(trifluoromethyl)phenyl]methoxy]ethyl]-3,6-dihydro-2H-pyridine-5-carboxylic acid;hydrochloride
|
| 别名 |
1-(2-(bis(4-(trifluoromethyl)phenyl)methoxy)ethyl)-1,2,5,6-tetrahydropyridine-3-carboxylic acid hydrochloride; 1-[2-[BIS[4-(TRIFLUOROMETHYL)PHENYL]METHOXY]ETHYL]-1,2,5,6-TETRAHYDROPYRIDINE-3-CARBOXYLIC ACID HYDROCHLORIDE; 1-{2-[Bis(4-(trifluoromethyl)phenyl)methoxy]ethyl}-1,2,5,6-tetrahydropyridine-3-carboxylic acid hydrochloride; 692-461-5; 110283-66-4; CI 966 hydrochloride; 3-Pyridinecarboxylic acid, 1,2,5,6-tetrahydro-1-(2-(bis(4-(trifluoromethyl)phenyl)methoxy)ethyl)-, hydrochloride; 3-Pyridinecarboxylicacid, 1-[2-[bis[4-(trifluoromethyl)phenyl]methoxy]ethyl]-1,2,5,6-tetrahydro-,hydrochloride (1:1);
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 50 mg/mL (98.06 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.90 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.90 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.90 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9613 mL | 9.8064 mL | 19.6128 mL | |
| 5 mM | 0.3923 mL | 1.9613 mL | 3.9226 mL | |
| 10 mM | 0.1961 mL | 0.9806 mL | 1.9613 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。